Electrochemical Generation of Cubic Shaped Nano Zn2SnO4 Photocatalysts
Corresponding Author: Chandrappa K. Govindappa
Nano-Micro Letters,
Vol. 5 No. 2 (2013), Article Number: 101-110
Abstract
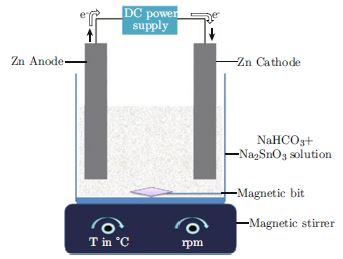
In this contribution, an efficient and simple two-step hybrid electrochemical-thermal route was developed for the synthesis of cubic shaped Zn2SnO4 (ZTO) nanoparticles using aqueous sodium bicarbonate (NaHCO3) and sodium stannate (Na2SnO3) electrolyte. The sacrificial Zn was used as anode and cathode in an undivided cell under galvanostatic mode at room temperature. The bath concentration and current density were respectively varied from 30 to 120 mmol and 0.05 to 1.5 A/dm2. The electrochemically generated precursor was calcined for an hour at different range of temperature from 60 to 600°C. The crystallite sizes in the range of 24-53 nm were calculated based on Debye-Scherrer equation. Scanning electron microscope and transmission electron microscopy results reveal that all the particles have cubic morphology with diameter of 40–50 nm. The as-prepared ZTO samples showed higher catalytic activity towards the degradation of methylene blue (MB) dye, and 90% degradation was found for the sample calcined at 600°C, which is greater than that of commercial TiO2-P25 photocatalysts. The photodegradation efficiency of ZTO samples was found to be a function of exposure time and the dye solution pH value. These results indicate that the ZTO nanoparticles may be employed to remove dyes from wastewater.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Y. K. Park, E. H. Tadd, M. Zubris and R. Tannenbaum, “Size-controlled synthesis of Alumina nanoparticles from aluminum alkoxides”, Mater. Res. Bull. 40(9), 1506–1512 (2005). http://dx.doi.org/10.1016/j.materresbull.2005.04.031
- M. R. Hoffmann, S. T. Martin, W. Y. Choi and D. W. Bahnemann, “Environmental applications of semiconductor Photocatalysis”, Chem. Rev. 95(1), 69–96 (1995). http://dx.doi.org/10.1021/cr00033a004
- T. Sehili, P. Boule and J. Lemaire, “Photocatalysed transformation of chloroaromatic derivatives on zinc oxide II-dichlorobenzenes”, J. Photochem. Photobio. A: Chem. 50(1), 103–116 (1989). http://dx.doi.org/10.1016/1010-6030(89)80024-3
- J. Villasenor, P. Reyes and G. Pecchi, “Photodegradation of pentachlorophenol on ZnO”, J. Chem. Tech. Biotech. 72(2), 105–110 (1998). http://dx.doi.org/10.1002/(SICI)1097–4660(199806)72:2<105::AID-JCTB883>3.3.CO;2-S
- M. D. Driessen, T. M. Miller and V. H. Grassian, “Photocatalytic oxidation of trichloroethylene on zinc oxide: characterization of surface-bound and gas-phase products and intermediates with FTIR spectroscopy”, J. Mol. Catal A: Chem. 131(1–3), 149–156 (1998). http://dx.doi.org/10.1016/S1381-1169(97)00262-8
- B. Tan, E. Toman, Y. G. Li and Y. Y. Wu, “Zinc stannate (Zn2SnO4) dye-sensitized solar cells”, J. Am. Chem. Soc. 129(14), 4162–4163 (2007). http://dx.doi.org/10.1021/ja070804f
- I. Stambolova, K. Konstantinov, D. Kovacheva, P. Peshev and T. Donchev, “Spray pyrolysis preparation and humidity sensing characteristics of spinel zinc stannate thin films”, J. Solid State Chem. 128(2), 305–309 (1997). http://dx.doi.org/10.1006/jssc.1996.7174
- J. H. Yu and G. M. Choi, “Current-voltage characteristics and selective CO detection of Zn2SnO4 and ZnO/Zn2SnO4, SnO2/Zn2SnO4 layered-type sensors”, Sens. Actuator B 72(2), 141–148 (2001). http://dx.doi.org/10.1016/S0925-4005(00)00642-0
- F. Belliard, P. A. Connor and J. T. S. Irvine, “Novel tin oxide-based anodes for Li-ion batteries”, Solid State Ionics. 135(1–4), 163–167 (2000). http://dx.doi.org/10.1016/S0167-2738(00)00296-4
- A. Rong, X. P. Gao, G. R. Li, T. Y. Yan, H. Y. Zhu, J. Q. Qu and D. Y. Song, “Hydrothermal synthesis of Zn2SnO4 as anode materials for Li-ion battery”, J. Phys. Chem. B 110(30), 14754–14760 (2006). http://dx.doi.org/10.1021/jp062875r
- X. D. Lou, X. H. Jia, J. Q. Xu, S. Z. Liu and Q. H. Gao, “Hydrothermal synthesis, characterization and photocatalytic properties of Zn2SnO4 nanocrystal”, Mater. Sci. Eng. A 432(1–2), 221–225 (2006). http://dx.doi.org/10.1016/j.msea.2006.06.010
- W. Cun, X. M. Wang, J. C. Zhao, B. X. Mai, G. Y. Sheng, P. A. Peng and J. M. Fu, “Synthesis, characterization and photocatalytic property of nano-sized Zn2SnO4”, J. Mater. Sci. 37(14), 2989–2996 (2002). http://dx.doi.org/10.1023/A:1016077216172
- S. Wang, Z. Yang, M. Lu, Y. Zhou, G. Zhou, Z. Qiu, S. Wang, H. Zhang and A. Zhang, “Coprecipitation synthesis of hollow Zn2SnO4 spheres”, Mater. Lett. 61, 3005–3008 (2007). http://dx.doi.org/10.1016/j.matlet.2006.07.197
- E. L. Foletto, S. L. Jahn and R. F. P. M. Moreira, “Hydrothermal preparation of Zn2SnO4 nanocrystals and photocatalytic degradation of a leather dye”, J. Appl. Electrochem. 40(14–15), 59–63 (2010). http://dx.doi.org/10.1007/s10800-009-9967-2
- X. Fu, X. Wang, J. Jong, Z. Ding, T. Yan, G. Zhang, Z. Zhang, H. Lin and X. Z. Fu, “Hydrothermal synthesis, characterization, and photocatalytic properties of Zn2SnO4”, J. Solid State Chem. 182(3), 517–524 (2009). http://dx.doi.org/10.1016/j.jssc.2008.11.029
- J. Zeng, M. D. Xin, K. W. Li, H. Wang, H. Yan and W. J. Zhang, “Transformation process and photocatalytic activities of hydrothermally synthesized Zn2SnO4 nanocrystals”, J. Phys. Chem. C 112(11), 4159–4167 (2008). http://dx.doi.org/10.1021/jp7113797
- Y. Lin, S. Lin, M. Luo and J. Liu, “Enhanced visible light photocatalytic activity of Zn2SnO4 via sulfur anion-doping”, Mater. Lett. 63(13–14), 1169–1171 (2009). http://dx.doi.org/10.1016/j.matlet.2009.02.020
- Yu Shiang Wu, Wen Ku Chang and Min Jou, “Photocatalytic analysis and characterization of Zn2SnO4 nanoparticles synthesized via hydrothermal method with Na2CO3 mineralizer”, Adv. Mater. Res. 97, 19–22 (2010). http://dx.doi.org/10.4028/www.scientific.net/AMR.97-101.19
- A. A. Firooz, A. R. Mahjouba, A. A. Khodadadi and M. Movahedi, “High photocatalytic activity of Zn2SnO4 among various nanostructures of Zn2xSn1-xO2 prepared by a hydrothermal method”, Chem. Eng. J. 165(2), 735–739 (2010). http://dx.doi.org/10.1016/j.cej.2010.09.052
- Z. Ai, S. Lee, Y. Huang, W. Ho and L. Zhang, “Photocatalytic removal of NO and HCHO over nanocrystalline Zn2SnO4 microcubes for indoor air purification”, J. Hazard. Mater. 179(1–3), 141–150 (2010). http://dx.doi.org/10.1016/j.jhazmat.2010.02.071
- M. J. Kim, S. H. Park and Y. D. Huh, “Photocatalytic activities of hydrothermally synthesized Zn2SnO4”, Bull. Korean Chem. Soc. 32(5), 1757–1760 (2011). http://dx.doi.org/10.5012/bkcs.2011.32.5.1757
- E. L. Foletto, J. M. Simoes, M. A. Mazutti, S. L. Jahn, E. I. Muller, L. S. F. Pereirab and E. M. M. Floresb, “Application of Zn2SnO4 photocatalyst prepared by microwave-assisted hydrothermal route in the degradation of organic pollutant under sunlight”, Ceramics Inter. 39(4), 4569–4574 (2013). http://dx.doi.org/10.1016/j.ceramint.2012.11.053
- H. Y. Chen, J. X. Wang, H. C. Yu, H. X. Yang, S. S. Xie and J. Q. Li, “Transmission electron microscopy study of pseudo periodically twinned Zn2SnO4 nanowires”, J. Phys. Chem. B 109(7), 2573–2577 (2005). http://dx.doi.org/10.1021/jp046125y
- J. S. Jie, G. Z. Wang, X. H. Han, J. P. Fang, Q. X. Yu, Y. Liao, B. Xu, Q. T. Wang and J. G. Hou, “Growth of ternary oxide nanowires by gold-catalyzed vapor-phase evaporation”, J. Phys. Chem. B 108(24), 8249–8253 (2004). http://dx.doi.org/10.1021/jp049230g
- M. Zhang, T. An, X. Hu, C. Wang, G. Sheng and J. Fu, “Preparation and photocatalytic properties of a nanometer ZnO-SnO2 coupled oxide”, Appl. Catal. A: Gen 260(2), 215–222 (2004). http://dx.doi.org/10.1016/j.apcata.2003.10.025
- G. Fu, H. Chen, Z. X. Chen, J. X. Zhang and H. Kohler, “Humidity sensing characteristics of Zn2SnO4-LiZnVO4 thick films prepared by the sol-gel method”, Sens. Actuator B 81(2–3), 308–312 (2002). http://dx.doi.org/10.1016/S0925-4005(01)00971-6
- A. Kurz, K. Brakecha, J. Puetz and M. A. Aegerter, “Strategies for novel transparent conducting solgel oxide coatings”, Thin Solid Films 502(1–2), 212–218 (2006). http://dx.doi.org/10.1016/j.tsf.2005.07.276
- N. Nikolic, Z. Marinkovic and T. Sreckovic, “The influence of grinding conditions on the mechanochemical synthesis of zinc stannate”, J. Mater. Sci. 39(16–17), 5239–5242 (2004). http://dx.doi.org/10.1023/B:JMSC.0000039218.82254.a4
- H. L. Zhu, D. R. Yang, G. X. Yu, H. Zhang, D. L. Jin and K. H. Yao, “Hydrothermal synthesis of Zn2SnO4 nanorods in the diameter regime of sub-5 nm and their properties”, J. Phys. Chem. B 110(15), 7631–7634 (2006). http://dx.doi.org/10.1021/jp060304t
- J. Fang, A. H. Huang, P. X. Zhu, N. S. Xu, J. Q. Xie, J. S. Chi, S. H. Feng, R. R. Xu and M. M. Wu, “Hydrothermal preparation and characterization of Zn2SnO4 particles”, Mater. Res. Bull. 36(7–8), 1391–1397 (2001). http://dx.doi.org/10.1016/S0025-5408(01)00621-3
- J. Zeng, M. D. Xin, K. W. Li, H. Wang, H. Yan and W. J. Zhang, “Transformation process and photocatalytic activities of hydrothermally synthesized Zn2SnO4 nanocrystals”, J. Phys. Chem. C 112(11), 4159–4167 (2008). http://dx.doi.org/10.1021/jp7113797
- E. Boschke, U. Bohmer, J. Lange, M. Constapel, M. Schellentrager and T. Bley, “The use of respirometric measurements to determine the toxicity of textile dyes in aqueous solution and after oxidative decolourisation processes”, Chemosphere. 67(11), 2163–2168 (2007). http://dx.doi.org/10.1016/j.chemosphere.2006.12.041
- H. Wang, C. Xie, W. Zhang, S. Cai, Z. Yang and Y. Gui, “Comparison of dye degradation efficiency using ZnO powders with various size scales”, J. Hazard. Mater. 141(3), 645–652 (2007). http://dx.doi.org/10.1016/j.jhazmat.2006.07.021
- R. Y. Hong, J. H. Li, L. L. Chen, D. Q. Liu, H. Z. Li, Y. Zheng and J. Ding, “Synthesis, surface modification and photocatalytic property of ZnO nanoparticles”, Powder Technology. 189(3), 426–432 (2009). http://dx.doi.org/10.1016/j.powtec.2008.07.004
- R. M. Trommer, A. K. Alves and C. P. Bergmann, “Synthesis, characterization and photocatalytic property of flame sprayed zinc oxide nanoparticles”, J. Alloys Compd. 491(1–2), 296–300 (2010). http://dx.doi.org/10.1016/j.jallcom.2009.10.147
- X. Ren, D. Han, D. Chen and F. Tang, “Large-scale synthesis of hexagonal cone-shaped ZnO nanoparticles with a simple route and their application to photocatalytic degradation”, Mater. Res. Bull. 42(5), 807–813 (2007). http://dx.doi.org/10.1016/j.materresbull.2006.08.030
- K. G. Chandrappa and T. V. Venkatesha, “Electrochemical Synthesis and photocatalytic property of zinc oxide nanoparticles”, Nano-Micro Lett. 4(1), 14–24 (2012). http://dx.doi.org/110.3786/nml.v4i1.p14-24
- K. G. Chandrappa, T. V. Venkatesha, K. Vathsala and C. Shivakumara, “A hybrid electrochemical-thermal method for the preparation of large ZnO nanoparticles”, J. Nanopart. Res. 12(7), 2667–2678 (2010). http://dx.doi.org/10.1007/s11051-009-9846-0
- P. Scherrer, Nachr. Ges. Wiss. Goettingen, Mathematical Physics K1 26, 98–100 (1918).
- C. N. R. Rao, “Chemical Applications of Infrared Spectroscopy”, Academic Press, New York-London (1963).
References
Y. K. Park, E. H. Tadd, M. Zubris and R. Tannenbaum, “Size-controlled synthesis of Alumina nanoparticles from aluminum alkoxides”, Mater. Res. Bull. 40(9), 1506–1512 (2005). http://dx.doi.org/10.1016/j.materresbull.2005.04.031
M. R. Hoffmann, S. T. Martin, W. Y. Choi and D. W. Bahnemann, “Environmental applications of semiconductor Photocatalysis”, Chem. Rev. 95(1), 69–96 (1995). http://dx.doi.org/10.1021/cr00033a004
T. Sehili, P. Boule and J. Lemaire, “Photocatalysed transformation of chloroaromatic derivatives on zinc oxide II-dichlorobenzenes”, J. Photochem. Photobio. A: Chem. 50(1), 103–116 (1989). http://dx.doi.org/10.1016/1010-6030(89)80024-3
J. Villasenor, P. Reyes and G. Pecchi, “Photodegradation of pentachlorophenol on ZnO”, J. Chem. Tech. Biotech. 72(2), 105–110 (1998). http://dx.doi.org/10.1002/(SICI)1097–4660(199806)72:2<105::AID-JCTB883>3.3.CO;2-S
M. D. Driessen, T. M. Miller and V. H. Grassian, “Photocatalytic oxidation of trichloroethylene on zinc oxide: characterization of surface-bound and gas-phase products and intermediates with FTIR spectroscopy”, J. Mol. Catal A: Chem. 131(1–3), 149–156 (1998). http://dx.doi.org/10.1016/S1381-1169(97)00262-8
B. Tan, E. Toman, Y. G. Li and Y. Y. Wu, “Zinc stannate (Zn2SnO4) dye-sensitized solar cells”, J. Am. Chem. Soc. 129(14), 4162–4163 (2007). http://dx.doi.org/10.1021/ja070804f
I. Stambolova, K. Konstantinov, D. Kovacheva, P. Peshev and T. Donchev, “Spray pyrolysis preparation and humidity sensing characteristics of spinel zinc stannate thin films”, J. Solid State Chem. 128(2), 305–309 (1997). http://dx.doi.org/10.1006/jssc.1996.7174
J. H. Yu and G. M. Choi, “Current-voltage characteristics and selective CO detection of Zn2SnO4 and ZnO/Zn2SnO4, SnO2/Zn2SnO4 layered-type sensors”, Sens. Actuator B 72(2), 141–148 (2001). http://dx.doi.org/10.1016/S0925-4005(00)00642-0
F. Belliard, P. A. Connor and J. T. S. Irvine, “Novel tin oxide-based anodes for Li-ion batteries”, Solid State Ionics. 135(1–4), 163–167 (2000). http://dx.doi.org/10.1016/S0167-2738(00)00296-4
A. Rong, X. P. Gao, G. R. Li, T. Y. Yan, H. Y. Zhu, J. Q. Qu and D. Y. Song, “Hydrothermal synthesis of Zn2SnO4 as anode materials for Li-ion battery”, J. Phys. Chem. B 110(30), 14754–14760 (2006). http://dx.doi.org/10.1021/jp062875r
X. D. Lou, X. H. Jia, J. Q. Xu, S. Z. Liu and Q. H. Gao, “Hydrothermal synthesis, characterization and photocatalytic properties of Zn2SnO4 nanocrystal”, Mater. Sci. Eng. A 432(1–2), 221–225 (2006). http://dx.doi.org/10.1016/j.msea.2006.06.010
W. Cun, X. M. Wang, J. C. Zhao, B. X. Mai, G. Y. Sheng, P. A. Peng and J. M. Fu, “Synthesis, characterization and photocatalytic property of nano-sized Zn2SnO4”, J. Mater. Sci. 37(14), 2989–2996 (2002). http://dx.doi.org/10.1023/A:1016077216172
S. Wang, Z. Yang, M. Lu, Y. Zhou, G. Zhou, Z. Qiu, S. Wang, H. Zhang and A. Zhang, “Coprecipitation synthesis of hollow Zn2SnO4 spheres”, Mater. Lett. 61, 3005–3008 (2007). http://dx.doi.org/10.1016/j.matlet.2006.07.197
E. L. Foletto, S. L. Jahn and R. F. P. M. Moreira, “Hydrothermal preparation of Zn2SnO4 nanocrystals and photocatalytic degradation of a leather dye”, J. Appl. Electrochem. 40(14–15), 59–63 (2010). http://dx.doi.org/10.1007/s10800-009-9967-2
X. Fu, X. Wang, J. Jong, Z. Ding, T. Yan, G. Zhang, Z. Zhang, H. Lin and X. Z. Fu, “Hydrothermal synthesis, characterization, and photocatalytic properties of Zn2SnO4”, J. Solid State Chem. 182(3), 517–524 (2009). http://dx.doi.org/10.1016/j.jssc.2008.11.029
J. Zeng, M. D. Xin, K. W. Li, H. Wang, H. Yan and W. J. Zhang, “Transformation process and photocatalytic activities of hydrothermally synthesized Zn2SnO4 nanocrystals”, J. Phys. Chem. C 112(11), 4159–4167 (2008). http://dx.doi.org/10.1021/jp7113797
Y. Lin, S. Lin, M. Luo and J. Liu, “Enhanced visible light photocatalytic activity of Zn2SnO4 via sulfur anion-doping”, Mater. Lett. 63(13–14), 1169–1171 (2009). http://dx.doi.org/10.1016/j.matlet.2009.02.020
Yu Shiang Wu, Wen Ku Chang and Min Jou, “Photocatalytic analysis and characterization of Zn2SnO4 nanoparticles synthesized via hydrothermal method with Na2CO3 mineralizer”, Adv. Mater. Res. 97, 19–22 (2010). http://dx.doi.org/10.4028/www.scientific.net/AMR.97-101.19
A. A. Firooz, A. R. Mahjouba, A. A. Khodadadi and M. Movahedi, “High photocatalytic activity of Zn2SnO4 among various nanostructures of Zn2xSn1-xO2 prepared by a hydrothermal method”, Chem. Eng. J. 165(2), 735–739 (2010). http://dx.doi.org/10.1016/j.cej.2010.09.052
Z. Ai, S. Lee, Y. Huang, W. Ho and L. Zhang, “Photocatalytic removal of NO and HCHO over nanocrystalline Zn2SnO4 microcubes for indoor air purification”, J. Hazard. Mater. 179(1–3), 141–150 (2010). http://dx.doi.org/10.1016/j.jhazmat.2010.02.071
M. J. Kim, S. H. Park and Y. D. Huh, “Photocatalytic activities of hydrothermally synthesized Zn2SnO4”, Bull. Korean Chem. Soc. 32(5), 1757–1760 (2011). http://dx.doi.org/10.5012/bkcs.2011.32.5.1757
E. L. Foletto, J. M. Simoes, M. A. Mazutti, S. L. Jahn, E. I. Muller, L. S. F. Pereirab and E. M. M. Floresb, “Application of Zn2SnO4 photocatalyst prepared by microwave-assisted hydrothermal route in the degradation of organic pollutant under sunlight”, Ceramics Inter. 39(4), 4569–4574 (2013). http://dx.doi.org/10.1016/j.ceramint.2012.11.053
H. Y. Chen, J. X. Wang, H. C. Yu, H. X. Yang, S. S. Xie and J. Q. Li, “Transmission electron microscopy study of pseudo periodically twinned Zn2SnO4 nanowires”, J. Phys. Chem. B 109(7), 2573–2577 (2005). http://dx.doi.org/10.1021/jp046125y
J. S. Jie, G. Z. Wang, X. H. Han, J. P. Fang, Q. X. Yu, Y. Liao, B. Xu, Q. T. Wang and J. G. Hou, “Growth of ternary oxide nanowires by gold-catalyzed vapor-phase evaporation”, J. Phys. Chem. B 108(24), 8249–8253 (2004). http://dx.doi.org/10.1021/jp049230g
M. Zhang, T. An, X. Hu, C. Wang, G. Sheng and J. Fu, “Preparation and photocatalytic properties of a nanometer ZnO-SnO2 coupled oxide”, Appl. Catal. A: Gen 260(2), 215–222 (2004). http://dx.doi.org/10.1016/j.apcata.2003.10.025
G. Fu, H. Chen, Z. X. Chen, J. X. Zhang and H. Kohler, “Humidity sensing characteristics of Zn2SnO4-LiZnVO4 thick films prepared by the sol-gel method”, Sens. Actuator B 81(2–3), 308–312 (2002). http://dx.doi.org/10.1016/S0925-4005(01)00971-6
A. Kurz, K. Brakecha, J. Puetz and M. A. Aegerter, “Strategies for novel transparent conducting solgel oxide coatings”, Thin Solid Films 502(1–2), 212–218 (2006). http://dx.doi.org/10.1016/j.tsf.2005.07.276
N. Nikolic, Z. Marinkovic and T. Sreckovic, “The influence of grinding conditions on the mechanochemical synthesis of zinc stannate”, J. Mater. Sci. 39(16–17), 5239–5242 (2004). http://dx.doi.org/10.1023/B:JMSC.0000039218.82254.a4
H. L. Zhu, D. R. Yang, G. X. Yu, H. Zhang, D. L. Jin and K. H. Yao, “Hydrothermal synthesis of Zn2SnO4 nanorods in the diameter regime of sub-5 nm and their properties”, J. Phys. Chem. B 110(15), 7631–7634 (2006). http://dx.doi.org/10.1021/jp060304t
J. Fang, A. H. Huang, P. X. Zhu, N. S. Xu, J. Q. Xie, J. S. Chi, S. H. Feng, R. R. Xu and M. M. Wu, “Hydrothermal preparation and characterization of Zn2SnO4 particles”, Mater. Res. Bull. 36(7–8), 1391–1397 (2001). http://dx.doi.org/10.1016/S0025-5408(01)00621-3
J. Zeng, M. D. Xin, K. W. Li, H. Wang, H. Yan and W. J. Zhang, “Transformation process and photocatalytic activities of hydrothermally synthesized Zn2SnO4 nanocrystals”, J. Phys. Chem. C 112(11), 4159–4167 (2008). http://dx.doi.org/10.1021/jp7113797
E. Boschke, U. Bohmer, J. Lange, M. Constapel, M. Schellentrager and T. Bley, “The use of respirometric measurements to determine the toxicity of textile dyes in aqueous solution and after oxidative decolourisation processes”, Chemosphere. 67(11), 2163–2168 (2007). http://dx.doi.org/10.1016/j.chemosphere.2006.12.041
H. Wang, C. Xie, W. Zhang, S. Cai, Z. Yang and Y. Gui, “Comparison of dye degradation efficiency using ZnO powders with various size scales”, J. Hazard. Mater. 141(3), 645–652 (2007). http://dx.doi.org/10.1016/j.jhazmat.2006.07.021
R. Y. Hong, J. H. Li, L. L. Chen, D. Q. Liu, H. Z. Li, Y. Zheng and J. Ding, “Synthesis, surface modification and photocatalytic property of ZnO nanoparticles”, Powder Technology. 189(3), 426–432 (2009). http://dx.doi.org/10.1016/j.powtec.2008.07.004
R. M. Trommer, A. K. Alves and C. P. Bergmann, “Synthesis, characterization and photocatalytic property of flame sprayed zinc oxide nanoparticles”, J. Alloys Compd. 491(1–2), 296–300 (2010). http://dx.doi.org/10.1016/j.jallcom.2009.10.147
X. Ren, D. Han, D. Chen and F. Tang, “Large-scale synthesis of hexagonal cone-shaped ZnO nanoparticles with a simple route and their application to photocatalytic degradation”, Mater. Res. Bull. 42(5), 807–813 (2007). http://dx.doi.org/10.1016/j.materresbull.2006.08.030
K. G. Chandrappa and T. V. Venkatesha, “Electrochemical Synthesis and photocatalytic property of zinc oxide nanoparticles”, Nano-Micro Lett. 4(1), 14–24 (2012). http://dx.doi.org/110.3786/nml.v4i1.p14-24
K. G. Chandrappa, T. V. Venkatesha, K. Vathsala and C. Shivakumara, “A hybrid electrochemical-thermal method for the preparation of large ZnO nanoparticles”, J. Nanopart. Res. 12(7), 2667–2678 (2010). http://dx.doi.org/10.1007/s11051-009-9846-0
P. Scherrer, Nachr. Ges. Wiss. Goettingen, Mathematical Physics K1 26, 98–100 (1918).
C. N. R. Rao, “Chemical Applications of Infrared Spectroscopy”, Academic Press, New York-London (1963).