5-Fluorouracil Loaded Chitosan–PVA/Na+MMT Nanocomposite Films for Drug Release and Antimicrobial Activity
Corresponding Author: A. Babul Reddy
Nano-Micro Letters,
Vol. 8 No. 3 (2016), Article Number: 260-269
Abstract
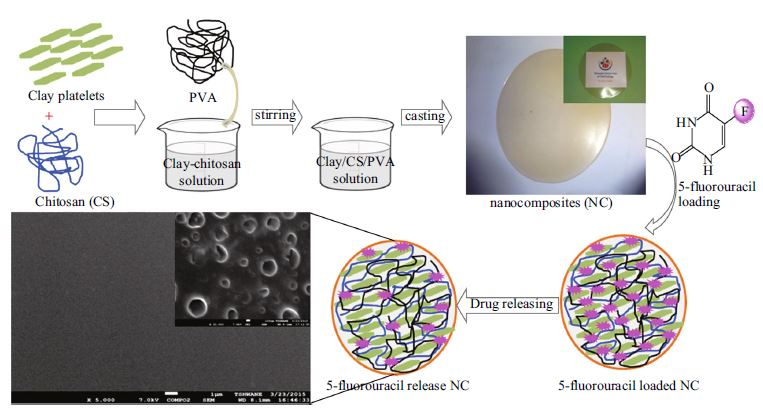
In the present study, chitosan and polyvinyl alcohol (PVA) were blended with different concentrations of sodium montmorillonite (Na+MMT) clay solution by a solvent casting method. X-ray diffraction and transition electron microscope results show that the film properties are related to the co-existence of Na+MMT intercalation/exfoliation in the blend and the interaction between chitosan–PVA and Na+MMT. 5-Fluorouracil (5-FU) was loaded with chitosan–PVA/Na+MMT nanocomposite films for in vitro drug delivery study. The antimicrobial activity of the chitosan–PVA/Na+MMT films showed significant effect against Salmonella (Gram-negative) and Staphylococcus aureus (Gram-positive), whereas 5-FU encapsulated chitosan–PVA/Na+MMT bio-nanocomposite films did not show any inhibition against bacteria. Our results indicate that combination of a flexible and soft polymeric material with high drug loading ability of a hard inorganic porous material can produce improved control over degradation and drug release. It will be an economically viable method for preparation of advanced drug delivery vehicles and biodegradable implants or scaffolds.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- K.E. Uhrich, S.M. Cannizzaro, R.S. Langer, K.M. Shakesheff, Polymeric systems for controlled drug release. Chem. Rev. 99(11), 3181–3198 (1999). doi:10.1021/cr940351u
- C. Aguzzi, P. Cerezo, C. Viseras, C. Caramella, Use of clays as drug delivery systems: possibilities and limitations. Appl. Clay Sci. 36(1–3), 22–36 (2007). doi:10.1016/j.clay.2006.06.015
- M. Rinaudo, Chitin and chitosan: properties and applications. Prog. Polym. Sci. 31(7), 603–632 (2006). doi:10.1016/j.progpolymsci.2006.06.001
- S.V. Madihally, H.W.T. Matthew, Porous chitosan scaffolds for tissue engineering. Biomaterials 20(12), 1133–1142 (1999). doi:10.1016/S0142-9612(99)00011-3
- F. Croisier, C. Jérôme, Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 49(4), 780–792 (2013). doi:10.1016/j.eurpolymj.2012.12.009
- T.M. Aminabhavi, H.G. Naik, Chemical compatibility study of geomembranes—sorption/desorption, diffusion and swelling phenomena. J. Hazard. Mater. 60(2), 175–203 (1998). doi:10.1016/S0304-3894(98)00090-9
- E.S. O’Sullivan, A. Vegas, D.G. Anderson, G.C. Weir, Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocr. Rev. 32(6), 827–844 (2011). doi:10.1210/er.2010-0026
- D.-H. Chen, J.-C. Leu, T.-C. Huang, Transport and hydrolysis of urea in a reactor–separator combining an anion-exchange membrane and immobilized urease. J. Chem. Technol. Biotechnol. 61(4), 351–357 (1994). doi:10.1002/jctb.280610411
- M.W. Sabaa, H.M. Abdallah, N.A. Mohamed, R.R. Mohamed, Synthesis, characterization and application of biodegradable crosslinked carboxymethyl chitosan/poly(vinyl alcohol) clay nanocomposites. Mater. Sci. Eng. C 56, 363–373 (2015). doi:10.1016/j.msec.2015.06.043
- J.K. Li, N. Wang, X.S. Wu, Poly(vinyl alcohol) nanoparticles prepared by freezing–thawing process for protein/peptide drug delivery. J. Control. Release 56(1–3), 117–126 (1998). doi:10.1016/S0168-3659(98)00089-3
- F. Yoshii, K. Makuuchi, D. Darwis, T. Iriawan, M.T. Razzak, J.M. Rosiak, Heat resistance poly(vinyl alcohol) hydrogel. Radiat. Phys. Chem. 46(2), 169–174 (1995). doi:10.1016/0969-806X(95)00008-L
- S.R. Stauffer, N.A. Peppast, Poly(vinyl alcohol) hydrogels prepared by freezing–thawing cyclic processing. Polymer 33(18), 3932–3936 (1992). doi:10.1016/0032-3861(92)90385-A
- B. Bolto, T. Tran, M. Hoang, Z. Xie, Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 34(9), 969–981 (2009). doi:10.1016/j.progpolymsci.2009.05.003
- G.V. Joshi, B.D. Kevadiya, H.A. Patel, H.C. Bajaj, R.V. Jasra, Montmorillonite as a drug delivery system: intercalation and in vitro release of timolol maleate. Int. J. Pharm. 374(1–2), 53–57 (2009). doi:10.1016/j.ijpharm.2009.03.004
- G.V. Joshi, H.A. Patel, B.D. Kevadiya, H.C. Bajaj, Montmorillonite intercalated with vitamin B1 as drug carrier. Appl. Clay Sci. 45(4), 248–253 (2009). doi:10.1016/j.clay.2009.06.001
- F. Bergaya, G. Lagaly (eds.), Handbook of Clay Science (Elsevier Science, Oxford, 2013)
- Y. Chen, A. Zhou, B. Liu, J. Liang, Tramadol hydrochloride/montmorillonite composite: preparation and controlled drug release. Appl. Clay Sci. 49(3), 108–112 (2010). doi:10.1016/j.clay.2010.04.011
- R.I. Iliescu, E. Andronescu, G. Voicu, A. Ficai, C.I. Covaliu, Hybrid materials based on montmorillonite and cytostatic drugs: preparation and characterization. Appl. Clay Sci. 52(1–2), 62–68 (2011). doi:10.1016/j.clay.2011.01.031
- S.-S. Feng, L. Mei, P. Anitha, C.W. Gan, W. Zhou, Poly(lactide)–vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel. Biomaterials 30(19), 3297–3306 (2009). doi:10.1016/j.biomaterials.2009.02.045
- Y. Dong, S.-S. Feng, Poly(d,l-lactide-co-glycolide)/montmorillonite nanoparticles for oral delivery of anticancer drugs. Biomaterials 26(30), 6068–6076 (2005). doi:10.1016/j.biomaterials.2005.03.021
- Y.-H. Lee, T.-F. Kuo, B.-Y. Chen, Y.-K. Feng, Y.-R. Wen, W.-C. Lin, F.H. Lin, Toxicity assessment of montmorillonite as a drug carrier for pharmaceutical applications: yeast and rats model. Biomed. Eng. 17(02), 72–78 (2005). doi:10.4015/S1016237205000111
- F.H. Lin, Y.H. Lee, C.H. Jian, J.-M. Wong, M.-J. Shieh, C.-Y. Wang, A study of purified montmorillonite intercalated with 5-fluorouracil as drug carrier. Biomaterials 23(9), 1981–1987 (2002). doi:10.1016/S0142-9612(01)00325-8
- C.D. Nunes, P.D. Vaz, A.C. Fernandes, P. Ferreira, C.C. Romão, M.J. Calhorda, Loading and delivery of sertraline using inorganic micro and mesoporous materials. Eur. J. Pharm. Biopharm. 66(3), 357–365 (2007). doi:10.1016/j.ejpb.2006.11.023
- Y. Seki, K. Yurdakoç, Adsorption of promethazine hydrochloride with KSF montmorillonite. Adsorption 12(1), 89–100 (2006). doi:10.1007/s10450-006-0141-4
- G.V. Joshi, B.D. Kevadiya, H.C. Bajaj, Design and evaluation of controlled drug delivery system of buspirone using inorganic layered clay mineral. Microporous Mesoporous Mater. 132(3), 526–530 (2010). doi:10.1016/j.micromeso.2010.04.003
- E. Healey, G.e. Stillfried, S. Eckermann, J.p. Dawber, P.r. Clingan, M. Ranson, Comparative effectiveness of 5-fluorouracil with and without oxaliplatin in the treatment of colorectal cancer in clinical practice. Anticancer Res. 33(3), 1053–1060 (2013), http://ar.iiarjournals.org/content/33/3/1053.long
- M. Osaki, S. Tatebe, A. Goto, H. Hayashi, M. Oshimura, H. Ito, 5-Fluorouracil (5-FU) induced apoptosis in gastric cancer cell lines: role of the p53 gene. Apoptosis 2(2), 221–226 (1997). doi:10.1023/a:1026476801463
- D.A. Cameron, H. Gabra, R.C. Leonard, Continuous 5-fluorouracil in the treatment of breast cancer. Br. J. Cancer 70(1), 120–124 (1994). doi:10.1038/bjc.1994.259
- H. Chen, W. Wu, Y. Li, T. Gong, X. Sun, Z. Zhang, A novel brain targeted 5-FU derivative with potential antitumor efficiency and decreased acute toxicity: synthesis, in vitro and in vivo evaluation. Die Pharmazie 69(4), 271–276 (2014). doi:10.1691/ph.2014.3200
- D. Ostertag, K.K. Amundson, F. Lopez Espinoza, B. Martin, T. Buckley et al., Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neurooncology 14(2), 145–159 (2012). doi:10.1093/neuonc/nor199
- J.M.G.H. van Riel, C.J. van Groeningen, S.H.M. Albers, M. Cazemier, S. Meijer, R. Bleichrodt, F.G. van den Berg, H.M. Pinedo, G. Giaccone, Hepatic arterial 5-fluorouracil in patients with liver metastases of colorectal cancer: single-centre experience in 145 patients. Ann. Oncol. 11(12), 1563–1570 (2000). doi: 10.1023/A:1008369520179
- S. Cascinu, R.R. Silva, S. Barni, R. Labianca, L. Frontini et al., A combination of gemcitabine and 5-fluorouracil in advanced pancreatic cancer, a report from the Italian Group for the Study of Digestive Tract Cancer (GISCAD). Br. J. Cancer 80(10), 1595–1598 (1999). doi:10.1038/sj.bjc.6690568
- S. Rao, D. Cunningham, Advanced pancreatic cancer—5 years on. Ann. Oncol. 13(8), 1165–1168 (2002). doi:10.1093/annonc/mdf313
- W.H. Isacoff, H.A. Reber, F.M. Purcell, B.M. Clerkin, K.M. Clerkin, Low-dose continuous infusion 5-fluorouracil combined with weekly leucovorin, nab-paclitaxel, oxaliplatin, and bevacizumab for patients with advanced pancreatic cancer: a pilot study. J. Clin. Oncol. (Meet. Abstr.) 28(15), e14545 (2010), http://meeting.ascopubs.org/cgi/content/abstract/28/15_suppl/e14545
- J. Nakano, C. Huang, D. Liu, D. Masuya, T. Nakashima, H. Yokomise, M. Ueno, H. Wada, M. Fukushima, Evaluations of biomarkers associated with 5-FU sensitivity for non-small-cell lung cancer patients postoperatively treated with UFT. Br. J. Cancer 95(5), 607–615 (2006). doi:10.1038/sj.bjc.6603297
- J.-G. Zhao, K.-M. Ren, J. Tang, Overcoming 5-FU resistance in human non-small cell lung cancer cells by the combination of 5-FU and cisplatin through the inhibition of glucose metabolism. Tumor Biol. 35(12), 12305–12315 (2014). doi:10.1007/s13277-014-2543-3
- T.J. Lynch, F. Kass, A.D. Elias, A. Skarin, E.F. Iii, L.A. Kalish, G. Strauss, L.N. Shulman, D.J. Sugarbaker, Cisplatin, 5-fluorouracil, and etoposide for advanced non-small cell lung cancer. Cancer 71(10), 2953–2957 (1993). doi:10.1002/1097-0142(19930515)71:10
- X. Pan, X. Zhang, H. Sun, J. Zhang, M. Yan, H. Zhang, Autophagy inhibition promotes 5-fluorouracil-induced apoptosis by stimulating ROS formation in human non-small cell lung cancer A549 cells. PLoS One 8(2), e56679 (2013). doi:10.1371/journal.pone.0056679
- B.D. Kevadiya, T.A. Patel, D.D. Jhala, R.P. Thumbar, H. Brahmbhatt et al., Layered inorganic nanocomposites: a promising carrier for 5-fluorouracil (5-FU). Eur. J. Pharm. Biopharm. 81(1), 91–101 (2012). doi:10.1016/j.ejpb.2012.01.004
- B. Van Triest, H.M. Pinedo, G. Giaccone, G.J. Peters, Downstream molecular determinants of response to 5-fluorouracil and antifolate thymidylate synthase inhibitors. Ann. Oncol. 11(4), 385–391 (2000), http://annonc.oxfordjournals.org/content/11/4/385
- D. Yohan, C. Cruje, X. Lu, D. Chithrani, Elucidating the uptake and distribution of nanoparticles in solid tumors via a multilayered cell culture model. Nano–Micro Lett. 7(2), 1–11 (2014). doi:10.1007/s40820-014-0025-1
- E. Aranda, E. Díaz-Rubio, A. Cervantes, A. Antón-Torres, A. Carrato et al., Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with weekly high-dose 48-hour continuous-infusion fluorouracil for advanced colorectal cancer: a Spanish Cooperative Group for Gastrointestinal Tumor Therapy (TTD) study. Ann. Oncol. 9(7), 727–731 (1998). doi:10.1023/A:1008282824860
- R.B. Diasio, Z. Lu, Dihydropyrimidine dehydrogenase activity and fluorouracil chemotherapy. J. Clin. Oncol. 12(11), 2239–2242 (1994), http://jco.ascopubs.org/content/12/11/2239
- E.C. Gamelin, E.M. Danquechin-Dorval, Y.F. Dumesnil, P.J. Maillart, M.J. Goudier et al., Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer 77(3), 441–451 (1996). doi:10.1002/(sici)1097-0142(19960201)77:3<441:aid-cncr4>3.0.co;2-n
- N.J. Meropol, D. Niedzwiecki, D. Hollis, R.L. Schilsky, R.J. Mayer, Cancer and The Leukemia Group B, Phase II study of oral eniluracil, 5-fluorouracil, and leucovorin in patients with advanced colorectal carcinoma. Cancer 91(7), 1256–1263 (2001). doi:10.1002/1097-0142(20010401)91:7<1256:aid-cncr1126>3.0.co;2-v
- S. Li, A. Wang, W. Jiang, Z. Guan, Pharmacokinetic characteristics and anticancer effects of 5-fluorouracil loaded nanoparticles. BMC Cancer 8, 103–103 (2008). doi:10.1186/1471-2407-8-103
- A.N.U. Parida, B. Binhani, P. Nayak, Synthesis and characterization of chitosan–polyvinyl alcohol blended with cloisite 30B for controlled release of the anticancer drug curcumin. J. Biomater. Nanobiotechnol. 2(4), 414–425 (2011). doi:10.4236/jbnb.2011.24051
- M.Y.K. Vimala, K. Varaprasad, N. Reddy, S. Ravindra, N. Naidu, K. Raju, Fabrication of curcumin encapsulated chitosan–PVA silver nanocomposite films for improved antimicrobial activity. J. Biomater. Nanobiotechnol. 2(1), 55–64 (2011). doi:10.4236/jbnb.2011.21008
- J.-W. Rhim, S.-I. Hong, H.-M. Park, P.K.W. Ng, Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 54(16), 5814–5822 (2006). doi:10.1021/jf060658h
- M. Koosha, H. Mirzadeh, M.A. Shokrgozar, M. Farokhi, Nanoclay-reinforced electrospun chitosan/PVA nanocomposite nanofibers for biomedical applications. RSC Adv. 5(14), 10479–10487 (2015). doi:10.1039/c4ra13972k
- G. Shen, Y. Guo, X. Sun, X. Wang, Electrochemical aptasensor based on prussian blue-chitosan–glutaraldehyde for the sensitive determination of tetracycline. Nano–-Micro Lett. 6(2), 143–152 (2014). doi:10.5101/nml.v6i2.p143-152
- M.D. Seema, Clay–polymer nanocomposites as a novel drug carrier: synthesis, characterization and controlled release study of propranolol hydrochloride. Appl. Clay Sci. 80–81, 85–92 (2013). doi:10.1016/j.clay.2013.06.009
- M.C. Costache, D. Wang, M.J. Heidecker, E. Manias, C.A. Wilkie, The thermal degradation of poly(methyl methacrylate) nanocomposites with montmorillonite, layered double hydroxides and carbon nanotubes. Polym. Adv. Technol. 17(4), 272–280 (2006). doi:10.1002/pat.697
- M. Kaci, C. Remili, A. Benhamida, S. Bruzaud, Y. Grohens, Recyclability of polystyrene/clay nanocomposites. Mol. Cryst. Liq. Cryst. 556(1), 94–106 (2012). doi:10.1080/15421406.2012.635922
- M. Kouchak, A. Ameri, B. Naseri, S. Kargar Boldaji, Chitosan and polyvinyl alcohol composite films containing nitrofurazone: preparation and evaluation. Iran. J. Basic Med. Sci. 17(1), 14–20 (2014), http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3938881/pdf/ijbms-17-014
References
K.E. Uhrich, S.M. Cannizzaro, R.S. Langer, K.M. Shakesheff, Polymeric systems for controlled drug release. Chem. Rev. 99(11), 3181–3198 (1999). doi:10.1021/cr940351u
C. Aguzzi, P. Cerezo, C. Viseras, C. Caramella, Use of clays as drug delivery systems: possibilities and limitations. Appl. Clay Sci. 36(1–3), 22–36 (2007). doi:10.1016/j.clay.2006.06.015
M. Rinaudo, Chitin and chitosan: properties and applications. Prog. Polym. Sci. 31(7), 603–632 (2006). doi:10.1016/j.progpolymsci.2006.06.001
S.V. Madihally, H.W.T. Matthew, Porous chitosan scaffolds for tissue engineering. Biomaterials 20(12), 1133–1142 (1999). doi:10.1016/S0142-9612(99)00011-3
F. Croisier, C. Jérôme, Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 49(4), 780–792 (2013). doi:10.1016/j.eurpolymj.2012.12.009
T.M. Aminabhavi, H.G. Naik, Chemical compatibility study of geomembranes—sorption/desorption, diffusion and swelling phenomena. J. Hazard. Mater. 60(2), 175–203 (1998). doi:10.1016/S0304-3894(98)00090-9
E.S. O’Sullivan, A. Vegas, D.G. Anderson, G.C. Weir, Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocr. Rev. 32(6), 827–844 (2011). doi:10.1210/er.2010-0026
D.-H. Chen, J.-C. Leu, T.-C. Huang, Transport and hydrolysis of urea in a reactor–separator combining an anion-exchange membrane and immobilized urease. J. Chem. Technol. Biotechnol. 61(4), 351–357 (1994). doi:10.1002/jctb.280610411
M.W. Sabaa, H.M. Abdallah, N.A. Mohamed, R.R. Mohamed, Synthesis, characterization and application of biodegradable crosslinked carboxymethyl chitosan/poly(vinyl alcohol) clay nanocomposites. Mater. Sci. Eng. C 56, 363–373 (2015). doi:10.1016/j.msec.2015.06.043
J.K. Li, N. Wang, X.S. Wu, Poly(vinyl alcohol) nanoparticles prepared by freezing–thawing process for protein/peptide drug delivery. J. Control. Release 56(1–3), 117–126 (1998). doi:10.1016/S0168-3659(98)00089-3
F. Yoshii, K. Makuuchi, D. Darwis, T. Iriawan, M.T. Razzak, J.M. Rosiak, Heat resistance poly(vinyl alcohol) hydrogel. Radiat. Phys. Chem. 46(2), 169–174 (1995). doi:10.1016/0969-806X(95)00008-L
S.R. Stauffer, N.A. Peppast, Poly(vinyl alcohol) hydrogels prepared by freezing–thawing cyclic processing. Polymer 33(18), 3932–3936 (1992). doi:10.1016/0032-3861(92)90385-A
B. Bolto, T. Tran, M. Hoang, Z. Xie, Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 34(9), 969–981 (2009). doi:10.1016/j.progpolymsci.2009.05.003
G.V. Joshi, B.D. Kevadiya, H.A. Patel, H.C. Bajaj, R.V. Jasra, Montmorillonite as a drug delivery system: intercalation and in vitro release of timolol maleate. Int. J. Pharm. 374(1–2), 53–57 (2009). doi:10.1016/j.ijpharm.2009.03.004
G.V. Joshi, H.A. Patel, B.D. Kevadiya, H.C. Bajaj, Montmorillonite intercalated with vitamin B1 as drug carrier. Appl. Clay Sci. 45(4), 248–253 (2009). doi:10.1016/j.clay.2009.06.001
F. Bergaya, G. Lagaly (eds.), Handbook of Clay Science (Elsevier Science, Oxford, 2013)
Y. Chen, A. Zhou, B. Liu, J. Liang, Tramadol hydrochloride/montmorillonite composite: preparation and controlled drug release. Appl. Clay Sci. 49(3), 108–112 (2010). doi:10.1016/j.clay.2010.04.011
R.I. Iliescu, E. Andronescu, G. Voicu, A. Ficai, C.I. Covaliu, Hybrid materials based on montmorillonite and cytostatic drugs: preparation and characterization. Appl. Clay Sci. 52(1–2), 62–68 (2011). doi:10.1016/j.clay.2011.01.031
S.-S. Feng, L. Mei, P. Anitha, C.W. Gan, W. Zhou, Poly(lactide)–vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel. Biomaterials 30(19), 3297–3306 (2009). doi:10.1016/j.biomaterials.2009.02.045
Y. Dong, S.-S. Feng, Poly(d,l-lactide-co-glycolide)/montmorillonite nanoparticles for oral delivery of anticancer drugs. Biomaterials 26(30), 6068–6076 (2005). doi:10.1016/j.biomaterials.2005.03.021
Y.-H. Lee, T.-F. Kuo, B.-Y. Chen, Y.-K. Feng, Y.-R. Wen, W.-C. Lin, F.H. Lin, Toxicity assessment of montmorillonite as a drug carrier for pharmaceutical applications: yeast and rats model. Biomed. Eng. 17(02), 72–78 (2005). doi:10.4015/S1016237205000111
F.H. Lin, Y.H. Lee, C.H. Jian, J.-M. Wong, M.-J. Shieh, C.-Y. Wang, A study of purified montmorillonite intercalated with 5-fluorouracil as drug carrier. Biomaterials 23(9), 1981–1987 (2002). doi:10.1016/S0142-9612(01)00325-8
C.D. Nunes, P.D. Vaz, A.C. Fernandes, P. Ferreira, C.C. Romão, M.J. Calhorda, Loading and delivery of sertraline using inorganic micro and mesoporous materials. Eur. J. Pharm. Biopharm. 66(3), 357–365 (2007). doi:10.1016/j.ejpb.2006.11.023
Y. Seki, K. Yurdakoç, Adsorption of promethazine hydrochloride with KSF montmorillonite. Adsorption 12(1), 89–100 (2006). doi:10.1007/s10450-006-0141-4
G.V. Joshi, B.D. Kevadiya, H.C. Bajaj, Design and evaluation of controlled drug delivery system of buspirone using inorganic layered clay mineral. Microporous Mesoporous Mater. 132(3), 526–530 (2010). doi:10.1016/j.micromeso.2010.04.003
E. Healey, G.e. Stillfried, S. Eckermann, J.p. Dawber, P.r. Clingan, M. Ranson, Comparative effectiveness of 5-fluorouracil with and without oxaliplatin in the treatment of colorectal cancer in clinical practice. Anticancer Res. 33(3), 1053–1060 (2013), http://ar.iiarjournals.org/content/33/3/1053.long
M. Osaki, S. Tatebe, A. Goto, H. Hayashi, M. Oshimura, H. Ito, 5-Fluorouracil (5-FU) induced apoptosis in gastric cancer cell lines: role of the p53 gene. Apoptosis 2(2), 221–226 (1997). doi:10.1023/a:1026476801463
D.A. Cameron, H. Gabra, R.C. Leonard, Continuous 5-fluorouracil in the treatment of breast cancer. Br. J. Cancer 70(1), 120–124 (1994). doi:10.1038/bjc.1994.259
H. Chen, W. Wu, Y. Li, T. Gong, X. Sun, Z. Zhang, A novel brain targeted 5-FU derivative with potential antitumor efficiency and decreased acute toxicity: synthesis, in vitro and in vivo evaluation. Die Pharmazie 69(4), 271–276 (2014). doi:10.1691/ph.2014.3200
D. Ostertag, K.K. Amundson, F. Lopez Espinoza, B. Martin, T. Buckley et al., Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neurooncology 14(2), 145–159 (2012). doi:10.1093/neuonc/nor199
J.M.G.H. van Riel, C.J. van Groeningen, S.H.M. Albers, M. Cazemier, S. Meijer, R. Bleichrodt, F.G. van den Berg, H.M. Pinedo, G. Giaccone, Hepatic arterial 5-fluorouracil in patients with liver metastases of colorectal cancer: single-centre experience in 145 patients. Ann. Oncol. 11(12), 1563–1570 (2000). doi: 10.1023/A:1008369520179
S. Cascinu, R.R. Silva, S. Barni, R. Labianca, L. Frontini et al., A combination of gemcitabine and 5-fluorouracil in advanced pancreatic cancer, a report from the Italian Group for the Study of Digestive Tract Cancer (GISCAD). Br. J. Cancer 80(10), 1595–1598 (1999). doi:10.1038/sj.bjc.6690568
S. Rao, D. Cunningham, Advanced pancreatic cancer—5 years on. Ann. Oncol. 13(8), 1165–1168 (2002). doi:10.1093/annonc/mdf313
W.H. Isacoff, H.A. Reber, F.M. Purcell, B.M. Clerkin, K.M. Clerkin, Low-dose continuous infusion 5-fluorouracil combined with weekly leucovorin, nab-paclitaxel, oxaliplatin, and bevacizumab for patients with advanced pancreatic cancer: a pilot study. J. Clin. Oncol. (Meet. Abstr.) 28(15), e14545 (2010), http://meeting.ascopubs.org/cgi/content/abstract/28/15_suppl/e14545
J. Nakano, C. Huang, D. Liu, D. Masuya, T. Nakashima, H. Yokomise, M. Ueno, H. Wada, M. Fukushima, Evaluations of biomarkers associated with 5-FU sensitivity for non-small-cell lung cancer patients postoperatively treated with UFT. Br. J. Cancer 95(5), 607–615 (2006). doi:10.1038/sj.bjc.6603297
J.-G. Zhao, K.-M. Ren, J. Tang, Overcoming 5-FU resistance in human non-small cell lung cancer cells by the combination of 5-FU and cisplatin through the inhibition of glucose metabolism. Tumor Biol. 35(12), 12305–12315 (2014). doi:10.1007/s13277-014-2543-3
T.J. Lynch, F. Kass, A.D. Elias, A. Skarin, E.F. Iii, L.A. Kalish, G. Strauss, L.N. Shulman, D.J. Sugarbaker, Cisplatin, 5-fluorouracil, and etoposide for advanced non-small cell lung cancer. Cancer 71(10), 2953–2957 (1993). doi:10.1002/1097-0142(19930515)71:10
X. Pan, X. Zhang, H. Sun, J. Zhang, M. Yan, H. Zhang, Autophagy inhibition promotes 5-fluorouracil-induced apoptosis by stimulating ROS formation in human non-small cell lung cancer A549 cells. PLoS One 8(2), e56679 (2013). doi:10.1371/journal.pone.0056679
B.D. Kevadiya, T.A. Patel, D.D. Jhala, R.P. Thumbar, H. Brahmbhatt et al., Layered inorganic nanocomposites: a promising carrier for 5-fluorouracil (5-FU). Eur. J. Pharm. Biopharm. 81(1), 91–101 (2012). doi:10.1016/j.ejpb.2012.01.004
B. Van Triest, H.M. Pinedo, G. Giaccone, G.J. Peters, Downstream molecular determinants of response to 5-fluorouracil and antifolate thymidylate synthase inhibitors. Ann. Oncol. 11(4), 385–391 (2000), http://annonc.oxfordjournals.org/content/11/4/385
D. Yohan, C. Cruje, X. Lu, D. Chithrani, Elucidating the uptake and distribution of nanoparticles in solid tumors via a multilayered cell culture model. Nano–Micro Lett. 7(2), 1–11 (2014). doi:10.1007/s40820-014-0025-1
E. Aranda, E. Díaz-Rubio, A. Cervantes, A. Antón-Torres, A. Carrato et al., Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with weekly high-dose 48-hour continuous-infusion fluorouracil for advanced colorectal cancer: a Spanish Cooperative Group for Gastrointestinal Tumor Therapy (TTD) study. Ann. Oncol. 9(7), 727–731 (1998). doi:10.1023/A:1008282824860
R.B. Diasio, Z. Lu, Dihydropyrimidine dehydrogenase activity and fluorouracil chemotherapy. J. Clin. Oncol. 12(11), 2239–2242 (1994), http://jco.ascopubs.org/content/12/11/2239
E.C. Gamelin, E.M. Danquechin-Dorval, Y.F. Dumesnil, P.J. Maillart, M.J. Goudier et al., Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer 77(3), 441–451 (1996). doi:10.1002/(sici)1097-0142(19960201)77:3<441:aid-cncr4>3.0.co;2-n
N.J. Meropol, D. Niedzwiecki, D. Hollis, R.L. Schilsky, R.J. Mayer, Cancer and The Leukemia Group B, Phase II study of oral eniluracil, 5-fluorouracil, and leucovorin in patients with advanced colorectal carcinoma. Cancer 91(7), 1256–1263 (2001). doi:10.1002/1097-0142(20010401)91:7<1256:aid-cncr1126>3.0.co;2-v
S. Li, A. Wang, W. Jiang, Z. Guan, Pharmacokinetic characteristics and anticancer effects of 5-fluorouracil loaded nanoparticles. BMC Cancer 8, 103–103 (2008). doi:10.1186/1471-2407-8-103
A.N.U. Parida, B. Binhani, P. Nayak, Synthesis and characterization of chitosan–polyvinyl alcohol blended with cloisite 30B for controlled release of the anticancer drug curcumin. J. Biomater. Nanobiotechnol. 2(4), 414–425 (2011). doi:10.4236/jbnb.2011.24051
M.Y.K. Vimala, K. Varaprasad, N. Reddy, S. Ravindra, N. Naidu, K. Raju, Fabrication of curcumin encapsulated chitosan–PVA silver nanocomposite films for improved antimicrobial activity. J. Biomater. Nanobiotechnol. 2(1), 55–64 (2011). doi:10.4236/jbnb.2011.21008
J.-W. Rhim, S.-I. Hong, H.-M. Park, P.K.W. Ng, Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 54(16), 5814–5822 (2006). doi:10.1021/jf060658h
M. Koosha, H. Mirzadeh, M.A. Shokrgozar, M. Farokhi, Nanoclay-reinforced electrospun chitosan/PVA nanocomposite nanofibers for biomedical applications. RSC Adv. 5(14), 10479–10487 (2015). doi:10.1039/c4ra13972k
G. Shen, Y. Guo, X. Sun, X. Wang, Electrochemical aptasensor based on prussian blue-chitosan–glutaraldehyde for the sensitive determination of tetracycline. Nano–-Micro Lett. 6(2), 143–152 (2014). doi:10.5101/nml.v6i2.p143-152
M.D. Seema, Clay–polymer nanocomposites as a novel drug carrier: synthesis, characterization and controlled release study of propranolol hydrochloride. Appl. Clay Sci. 80–81, 85–92 (2013). doi:10.1016/j.clay.2013.06.009
M.C. Costache, D. Wang, M.J. Heidecker, E. Manias, C.A. Wilkie, The thermal degradation of poly(methyl methacrylate) nanocomposites with montmorillonite, layered double hydroxides and carbon nanotubes. Polym. Adv. Technol. 17(4), 272–280 (2006). doi:10.1002/pat.697
M. Kaci, C. Remili, A. Benhamida, S. Bruzaud, Y. Grohens, Recyclability of polystyrene/clay nanocomposites. Mol. Cryst. Liq. Cryst. 556(1), 94–106 (2012). doi:10.1080/15421406.2012.635922
M. Kouchak, A. Ameri, B. Naseri, S. Kargar Boldaji, Chitosan and polyvinyl alcohol composite films containing nitrofurazone: preparation and evaluation. Iran. J. Basic Med. Sci. 17(1), 14–20 (2014), http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3938881/pdf/ijbms-17-014