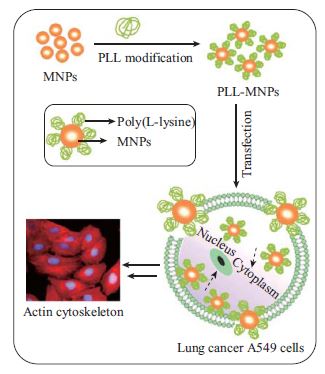
Highly Efficient Labeling of Human Lung Cancer Cells Using Cationic Poly-l-lysine-Assisted Magnetic Iron Oxide Nanoparticles
Corresponding Author: Xueqin Wang
Nano-Micro Letters,
Vol. 7 No. 4 (2015), Article Number: 374-384
Abstract
Cell labeling with magnetic iron oxide nanoparticles (IONPs) is increasingly a routine approach in the cell-based cancer treatment. However, cell labeling with magnetic IONPs and their leading effects on the biological properties of human lung carcinoma cells remain scarcely reported. Therefore, in the present study the magnetic γ-Fe2O3 nanoparticles (MNPs) were firstly synthesized and surface-modified with cationic poly-l-lysine (PLL) to construct the PLL-MNPs, which were then used to magnetically label human A549 lung cancer cells. Cell viability and proliferation were evaluated with propidium iodide/fluorescein diacetate double staining and standard 3-(4,5-dimethylthiazol-2-diphenyl-tetrazolium) bromide assay, and the cytoskeleton was immunocytochemically stained. The cell cycle of the PLL-MNP-labeled A549 lung cancer cells was analyzed using flow cytometry. Apoptotic cells were fluorescently analyzed with nuclear-specific staining after the PLL-MNP labeling. The results showed that the constructed PLL-MNPs efficiently magnetically labeled A549 lung cancer cells and that, at low concentrations, labeling did not affect cellular viability, proliferation capability, cell cycle, and apoptosis. Furthermore, the cytoskeleton in the treated cells was detected intact in comparison with the untreated counterparts. However, the results also showed that at high concentration (400 µg mL−1), the PLL-MNPs would slightly impair cell viability, proliferation, cell cycle, and apoptosis and disrupt the cytoskeleton in the treated A549 lung cancer cells. Therefore, the present results indicated that the PLL-MNPs at adequate concentrations can be efficiently used for labeling A549 lung cancer cells and could be considered as a feasible approach for magnetic targeted anti-cancer drug/gene delivery, targeted diagnosis, and therapy in lung cancer treatment.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- R. Siegel, D. Naishadham, A. Jemal, Cancer statistics. CA Cancer J. Clin. 62(1), 10–29 (2012). doi:10.3322/caac.20138
- P. Leidinger, A. Keller, S. Heisel, N. Ludwig, S. Rheinheimer et al., Identification of lung cancer with high sensitivity and specificity by blood testing. Respir. Res. 11, 18 (2010). doi:10.1186/1465-9921-11-18
- Z. Lwin, J.W. Riess, D. Gandara, The continuing role of chemotherapy for advanced non-small cell lung cancer in the targeted therapy era. J. Thorac. Dis. 5(Suppl 5), S556–S564 (2013)
- S.J. Choi, J.M. Oh, J.H. Choy, Toxicological effects of inorganic nanoparticles on human lung cancer A549 cells. J. Inorg. Biochem. 103(3), 463–471 (2009). doi:10.1016/j.jinorgbio.2008.12.017
- P. Dames, B. Gleich, A. Flemmer, K. Hajek, N. Seidl, F. Wiekhorst, D. Eberbeck, I. Bittmann, C. Bergemann, T. Weyh, L. Trahms, J. Rosenecker, C. Rudolph, Targeted delivery of magnetic aerosol droplets to the lung. Nat. Nanotechnol. 2(8), 495–499 (2007). doi:10.1038/nnano.2007.217
- Chenchen Bao, Lei Chen, Tao Wang, Chong Lei, Furong Tian, Daxiang Cui, Yong Zhou, One step quick detection of cancer cell surface marker by integrated NiFe-based magnetic biosensing cell cultural chip. Nano-Micro Lett. 5(3), 213–222 (2013). doi:10.5101/nml.v5i3.p213-222
- K.S. Tang, S.M. Hashmi, E.M. Shapiro, The effect of cryoprotection on the use of PLGA encapsulated iron oxide nanoparticles for magnetic cell labeling. Nanotechnology 24(12), 125101 (2013). doi:10.1088/0957-4484/24/12/125101
- E. Karaoğlu, H. Kavas, A. Baykal, M.S. Toprak, H. Sözeri, Effect of hydrolyzing agents on the properties of poly(ethylene glycol)-Fe3O4 nanocomposite. Nano-Micro Lett. 3(2), 79–85 (2011). doi:10.3786/nml.v3i2.p79-85
- X. Wang, F. Wei, S. Yan, H. Zhang, X. Tan et al., Innovative fluorescent magnetic albumin microbead-assisted cell labeling and intracellular imaging of glioblastoma cells. Biosens. Bioelectron. 54C, 55–63 (2014). doi:10.1016/j.bios.2013.10.041
- J.R. McCarthy, R. Weissleder, Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 60(11), 1241–1251 (2008). doi:10.1016/j.addr.2008.03.014
- M. Muthana, S.D. Scott, N. Farrow, F. Morrow, C. Murdoch, S. Grubb, N. Brown, J. Dobson, C.E. Lewis, A novel magnetic approach to enhance the efficacy of cell-based gene therapies. Gene Ther. 15(12), 902–910 (2008). doi:10.1038/gt.2008.57
- C. Trueck, K. Zimmermann, O. Mykhaylyk, M. Anton, S. Vosen, D. Wenzel, B.K. Fleischmann, A. Pfeifer, Optimization of magnetic nanoparticle-assisted lentiviral gene transfer. Pharm. Res. 29(5), 1255–1269 (2012). doi:10.1007/s11095-011-0660-x
- S. Panseri, C. Cunha, T. D’Alessandro, M. Sandri, A. Russo, G. Giavaresi, M. Marcacci, C.T. Hung, A. Tampieri, Magnetic hydroxyapatite bone substitutes to enhance tissue regeneration: evaluation in vitro using osteoblast-like cells and in vivo in a bone defect. PLoS One 7(6), e38710 (2012). doi:10.1371/journal.pone.0038710
- R. Sensenig, Y. Sapir, C. MacDonald, S. Cohen, B. Polyak, Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomedicine (Lond.) 7(9), 1425–1442 (2012). doi:10.2217/nnm.12.109
- H.J. Hathaway, K.S. Butler, N.L. Adolphi, D.M. Lovato, R. Belfon et al., Detection of breast cancer cells using targeted magnetic nanoparticles and ultra-sensitive magnetic field sensors. Breast Cancer Res. 13(5), R108 (2011). doi:10.1186/bcr3050
- A. Pourjavadi, S.H. Hosseini, M. Alizadeh, C. Bennett, Magnetic pH-responsive nanocarrier with long spacer length and high colloidal stability for controlled delivery of doxorubicin. Colloids Surf. B. Biointerfaces 116, 49–54 (2014). doi:10.1016/j.colsurfb.2013.12.048
- O. Veiseh, J.W. Gunn, M. Zhang, Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 62(3), 284–304 (2010). doi:10.1016/j.addr.2009.11.002
- C.H. Wu, Y.Y. Huang, P. Chen, K. Hoshino, H. Liu, E.P. Frenkel, J.X. Zhang, K.V. Sokolov, Versatile immunomagnetic nanocarrier platform for capturing cancer cells. ACS Nano 7(10), 8816–8823 (2013). doi:10.1021/nn403281e
- R. Kamali, S.A. Shekoohi, A. Binesh, Effects of magnetic particles entrance arrangements on mixing efficiency of a magnetic bead micromixer. Nano-Micro Lett. 6(1), 30–37 (2014). doi:10.5101/nml.v6i1.p30-37
- P. Smirnov, Cellular magnetic resonance imaging using superparamagnetic anionic iron oxide nanoparticles: applications to in vivo trafficking of lymphocytes and cell-based anticancer therapy. Methods Mol. Biol. 512, 333–353 (2009). doi:10.1007/978-1-60327-530-9_19
- X. Wu, Y. Tan, H. Mao, M. Zhang, Toxic effects of iron oxide nanoparticles on human umbilical vein endothelial cells. Int. J. Nanomedicine 5, 385–399 (2010). doi:10.2147/IJN.S10458
- H. Xu, Z.P. Aguilar, L. Yang, M. Kuang, H. Duan, Y. Xiong, H. Wei, A. Wang, Antibody conjugated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood. Biomaterials 32(36), 9758–9765 (2011). doi:10.1016/j.biomaterials.2011.08.076
- H. Ding, V. Sagar, M. Agudelo, S. Pilakka-Kanthikeel, V.S. Atluri, A. Raymond, T. Samikkannu, M.P. Nair, Enhanced blood-brain barrier transmigration using a novel transferrin embedded fluorescent magneto-liposome nanoformulation. Nanotechnology 25(5), 055101 (2014). doi:10.1088/0957-4484/25/5/055101
- M. Kumar, G. Singh, V. Arora, S. Mewar, U. Sharma, N.R. Jagannathan, S. Sapra, A.K. Dinda, S. Kharbanda, H. Singh, Cellular interaction of folic acid conjugated superparamagnetic iron oxide nanoparticles and its use as contrast agent for targeted magnetic imaging of tumor cells. Int. J. Nanomedicine 7, 3503–3516 (2012)
- M. Arsianti, M. Lim, C.P. Marquis, R. Amal, Assembly of polyethylenimine-based magnetic iron oxide vectors: insights into gene delivery. Langmuir 26(10), 7314–7326 (2010). doi:10.1021/la9041919
- R. Namgung, K. Singha, M.K. Yu, S. Jon, Y.S. Kim, Y. Ahn, I.K. Park, W.J. Kim, Hybrid superparamagnetic iron oxide nanoparticle-branched polyethylenimine magnetoplexes for gene transfection of vascular endothelial cells. Biomaterials 31(14), 4204–4213 (2010). doi:10.1016/j.biomaterials.2010.01.123
- S. Jiang, A.A. Eltoukhy, K.T. Love, R. Langer, D.G. Anderson, Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Lett. 13(3), 1059–1064 (2013). doi:10.1021/nl304287a
- S.J. Soenen, S.C. De Smedt, K. Braeckmans, Limitations and caveats of magnetic cell labeling using transfection agent complexed iron oxide nanoparticles. Contrast Media Mol. Imaging 7(2), 140–152 (2012). doi:10.1002/cmmi.472
- M. Babic, D. Horák, M. Trchová, P. Jendelová, K. Glogarová, P. Lesný, V. Herynek, M. Hájek, E. Syková, Poly(l-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjug. Chem. 19(3), 740–750 (2008). doi:10.1021/bc700410z
- X. Wang, Q. Tu, B. Zhao, Y. An, J.C. Wang, W. Liu, M.S. Yuan, S.M. Ahmed, J. Xu, R. Liu, Y. Zhang, J. Wang, Effects of poly(l-lysine)-modified Fe3O4 nanoparticles on endogenous reactive oxygen species in cancer stem cells. Biomaterials 34(4), 1155–1169 (2013). doi:10.1016/j.biomaterials.2012.10.063
- S. Shanehsazzadeh, M.A. Oghabian, B.J. Allen, M. Amanlou, A. Masoudi, F.J. Daha, Evaluating the effect of ultrasmall superparamagnetic iron oxide nanoparticles for a long-term magnetic cell labeling. J. Med. Phys. 38(1), 34–40 (2013). doi:10.4103/0971-6203.106603
- Z. Wang, A. Cuschieri, Tumour cell labelling by magnetic nanoparticles with determination of intracellular iron content and spatial distribution of the intracellular iron. Int. J. Mol. Sci. 14(5), 9111–9125 (2013). doi:10.3390/ijms14059111
- D. Mazia, G. Schatten, W. Sale, Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J. Cell Biol. 66(1), 198–200 (1975). doi:10.1083/jcb.66.1.198
- M. Watanabe, M. Yoneda, A. Morohashi, Y. Hori, D. Okamoto et al., Effects of Fe3O4 magnetic nanoparticles on A549 cells. Int. J. Mol. Sci. 14(8), 15546–15560 (2013). doi:10.3390/ijms140815546
- A. Akbarzadeh, M. Samiei, S.W. Joo, M. Anzaby, Y. Hanifehpour, H.T. Nasrabadi, S. Davaran, Synthesis, characterization and in vitro studies of doxorubicin-loaded magnetic nanoparticles grafted to smart copolymers on A549 lung cancer cell line. J. Nanobiotechnology 10, 46 (2012). doi:10.1186/1477-3155-10-46
- S. Qu, H. Yang, D. Ren, S. Kan, G. Zou, D. Li, M. Li, Magnetite nanoparticles prepared by precipitation from partially reduced ferric chloride aqueous solutions. J. Colloid Interface Sci. 215(1), 190–192 (1999). doi:10.1006/jcis.1999.6185
- Y.K. Sun, M. Ma, Y. Zhang, N. Gu, Synthesis of nanometer-size maghemite particles from magnetite. Colloids Surf. A. Physicochem Eng. Asp. 245(1–3), 15–19 (2004). doi:10.1016/j.colsurfa.2004.05.009
- X. Wang, F. Wei, A. Liu, L. Wang, J.C. Wang, L. Ren, W. Liu, Q. Tu, L. Li, J. Wang, Cancer stem cell labeling using poly(l-lysine)-modified iron oxide nanoparticles. Biomaterials 33(14), 3719–3732 (2012). doi:10.1016/j.biomaterials.2012.01.058
- B.W. Kristensen, H. Noer, J.B. Gramsbergen, J. Zimmer, J. Noraberg, Colchicine induces apoptosis in organotypic hippocampal slice cultures. Brain Res. 964(2), 264–278 (2003). doi:10.1016/S0006-8993(02)04080-5
- X. Huang, L. Li, Q. Tu, J. Wang, W. Liu, X. Wang, L. Ren, J. Wang, On-chip cell migration assay for quantifying the effect of ethanol on MCF-7 human breast cancer cells. Microfluid. Nanofluidics 10(6), 1333–1341 (2011). doi:10.1007/s10404-011-0766-9
- A.S. Arbab, G.T. Yocum, H. Kalish, E.K. Jordan, S.A. Anderson, A.Y. Khakoo, E.J. Read, J.A. Frank, Efficient magnetic cell labeling with protamine sulfate complexed to ferum-oxides for cellular MRI. Blood 104(4), 1217–1223 (2004). doi:10.1182/blood-2004-02-0655
- D. Horák, M. Babič, P. Jendelová, V. Herynek, M. Trchová, Z. Pientka, E. Pollert, M. Hájek, E. Syková, d-mannose-modified iron oxide nanoparticles for stem cell labeling. Bioconjug. Chem. 18(3), 635–644 (2007). doi:10.1021/bc060186c
- J. Wang, X. Wang, L. Ren, Q. Wang, L. Li et al., Conjugation of biomolecules with magnetic protein microspheres for the assay of early biomarkers associated with acute myocardial infarction. Anal. Chem. 81(15), 6210–6217 (2009). doi:10.1021/ac9007418
- M. Neri, C. Maderna, C. Cavazzin, V. Deidda-Vigoriti, L.S. Politi et al., Efficient in vitro labeling of human neural precursor cells with superparamagnetic iron oxide particles: relevance for in vivo cell tracking. Stem Cells 26(2), 505–516 (2008). doi:10.1634/stemcells.2007-0251
- S. Naqvi, M. Samim, M. Abdin, F.J. Ahmed, A. Maitra, C. Prashant, A.K. Dinda, Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomedicine 5, 983–989 (2010). doi:10.2147/IJN.S13244
- S.J. Soenen, E. Illyes, D. Vercauteren, K. Braeckmans, Z. Majer, S.C. De Smedt, M. De Cuyper, The role of nanoparticle concentration-dependent induction of cellular stress in the internalization of non-toxic cationic magnetoliposomes. Biomaterials 30(36), 6803–6813 (2009). doi:10.1016/j.biomaterials.2009.08.050
- S.J. Soenen, U. Himmelreich, N. Nuytten, M. De Cuyper, Cytotoxic effects of iron oxide nanoparticles and implications for safety in cell labeling. Biomaterials 32(1), 195–205 (2011). doi:10.1016/j.biomaterials.2010.08.075
- A.H. Koyama, A. Adachi, Induction of apoptosis by herpes simplex virus type 1. J. Gen. Virol. 78(Pt 11), 2909–2912 (1997)
- A.H. Koyama, Y. Miwa, Suppression of apoptotic DNA fragmentation in herpes simplex virus type 1-infected cells. J. Virol. 71(3), 2567–2571 (1997)
- S. Elmore, Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35, 495–516 (2007). doi:10.1080/01926230701320337
- S.R. Denmeade, J.T. Isaacs, Programmed cell death (apoptosis) and cancer chemotherapy. Cancer Control 3(4), 303–309 (1996)
- K.F. Jorgenson, U. Varshney, J.H. van de Sande, Interaction of Hoechst 33258 with repeating synthetic DNA polymers and natural DNA. J. Biomol. Struct. Dyn. 5(5), 1005–1023 (1988). doi:10.1080/07391102.1988.10506446
- S.Y. Breusegem, R.M. Clegg, F.G. Loontiens, Base-sequence specificity of Hoechst 33258 and DAPI binding to five (A/T)4 DNA sites with kinetic evidence for more than one high-affinity Hoechst 33258-AATT complex. J. Mol. Biol. 315(5), 1049–1061 (2002). doi:10.1006/jmbi.2001.5301
References
R. Siegel, D. Naishadham, A. Jemal, Cancer statistics. CA Cancer J. Clin. 62(1), 10–29 (2012). doi:10.3322/caac.20138
P. Leidinger, A. Keller, S. Heisel, N. Ludwig, S. Rheinheimer et al., Identification of lung cancer with high sensitivity and specificity by blood testing. Respir. Res. 11, 18 (2010). doi:10.1186/1465-9921-11-18
Z. Lwin, J.W. Riess, D. Gandara, The continuing role of chemotherapy for advanced non-small cell lung cancer in the targeted therapy era. J. Thorac. Dis. 5(Suppl 5), S556–S564 (2013)
S.J. Choi, J.M. Oh, J.H. Choy, Toxicological effects of inorganic nanoparticles on human lung cancer A549 cells. J. Inorg. Biochem. 103(3), 463–471 (2009). doi:10.1016/j.jinorgbio.2008.12.017
P. Dames, B. Gleich, A. Flemmer, K. Hajek, N. Seidl, F. Wiekhorst, D. Eberbeck, I. Bittmann, C. Bergemann, T. Weyh, L. Trahms, J. Rosenecker, C. Rudolph, Targeted delivery of magnetic aerosol droplets to the lung. Nat. Nanotechnol. 2(8), 495–499 (2007). doi:10.1038/nnano.2007.217
Chenchen Bao, Lei Chen, Tao Wang, Chong Lei, Furong Tian, Daxiang Cui, Yong Zhou, One step quick detection of cancer cell surface marker by integrated NiFe-based magnetic biosensing cell cultural chip. Nano-Micro Lett. 5(3), 213–222 (2013). doi:10.5101/nml.v5i3.p213-222
K.S. Tang, S.M. Hashmi, E.M. Shapiro, The effect of cryoprotection on the use of PLGA encapsulated iron oxide nanoparticles for magnetic cell labeling. Nanotechnology 24(12), 125101 (2013). doi:10.1088/0957-4484/24/12/125101
E. Karaoğlu, H. Kavas, A. Baykal, M.S. Toprak, H. Sözeri, Effect of hydrolyzing agents on the properties of poly(ethylene glycol)-Fe3O4 nanocomposite. Nano-Micro Lett. 3(2), 79–85 (2011). doi:10.3786/nml.v3i2.p79-85
X. Wang, F. Wei, S. Yan, H. Zhang, X. Tan et al., Innovative fluorescent magnetic albumin microbead-assisted cell labeling and intracellular imaging of glioblastoma cells. Biosens. Bioelectron. 54C, 55–63 (2014). doi:10.1016/j.bios.2013.10.041
J.R. McCarthy, R. Weissleder, Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 60(11), 1241–1251 (2008). doi:10.1016/j.addr.2008.03.014
M. Muthana, S.D. Scott, N. Farrow, F. Morrow, C. Murdoch, S. Grubb, N. Brown, J. Dobson, C.E. Lewis, A novel magnetic approach to enhance the efficacy of cell-based gene therapies. Gene Ther. 15(12), 902–910 (2008). doi:10.1038/gt.2008.57
C. Trueck, K. Zimmermann, O. Mykhaylyk, M. Anton, S. Vosen, D. Wenzel, B.K. Fleischmann, A. Pfeifer, Optimization of magnetic nanoparticle-assisted lentiviral gene transfer. Pharm. Res. 29(5), 1255–1269 (2012). doi:10.1007/s11095-011-0660-x
S. Panseri, C. Cunha, T. D’Alessandro, M. Sandri, A. Russo, G. Giavaresi, M. Marcacci, C.T. Hung, A. Tampieri, Magnetic hydroxyapatite bone substitutes to enhance tissue regeneration: evaluation in vitro using osteoblast-like cells and in vivo in a bone defect. PLoS One 7(6), e38710 (2012). doi:10.1371/journal.pone.0038710
R. Sensenig, Y. Sapir, C. MacDonald, S. Cohen, B. Polyak, Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomedicine (Lond.) 7(9), 1425–1442 (2012). doi:10.2217/nnm.12.109
H.J. Hathaway, K.S. Butler, N.L. Adolphi, D.M. Lovato, R. Belfon et al., Detection of breast cancer cells using targeted magnetic nanoparticles and ultra-sensitive magnetic field sensors. Breast Cancer Res. 13(5), R108 (2011). doi:10.1186/bcr3050
A. Pourjavadi, S.H. Hosseini, M. Alizadeh, C. Bennett, Magnetic pH-responsive nanocarrier with long spacer length and high colloidal stability for controlled delivery of doxorubicin. Colloids Surf. B. Biointerfaces 116, 49–54 (2014). doi:10.1016/j.colsurfb.2013.12.048
O. Veiseh, J.W. Gunn, M. Zhang, Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 62(3), 284–304 (2010). doi:10.1016/j.addr.2009.11.002
C.H. Wu, Y.Y. Huang, P. Chen, K. Hoshino, H. Liu, E.P. Frenkel, J.X. Zhang, K.V. Sokolov, Versatile immunomagnetic nanocarrier platform for capturing cancer cells. ACS Nano 7(10), 8816–8823 (2013). doi:10.1021/nn403281e
R. Kamali, S.A. Shekoohi, A. Binesh, Effects of magnetic particles entrance arrangements on mixing efficiency of a magnetic bead micromixer. Nano-Micro Lett. 6(1), 30–37 (2014). doi:10.5101/nml.v6i1.p30-37
P. Smirnov, Cellular magnetic resonance imaging using superparamagnetic anionic iron oxide nanoparticles: applications to in vivo trafficking of lymphocytes and cell-based anticancer therapy. Methods Mol. Biol. 512, 333–353 (2009). doi:10.1007/978-1-60327-530-9_19
X. Wu, Y. Tan, H. Mao, M. Zhang, Toxic effects of iron oxide nanoparticles on human umbilical vein endothelial cells. Int. J. Nanomedicine 5, 385–399 (2010). doi:10.2147/IJN.S10458
H. Xu, Z.P. Aguilar, L. Yang, M. Kuang, H. Duan, Y. Xiong, H. Wei, A. Wang, Antibody conjugated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood. Biomaterials 32(36), 9758–9765 (2011). doi:10.1016/j.biomaterials.2011.08.076
H. Ding, V. Sagar, M. Agudelo, S. Pilakka-Kanthikeel, V.S. Atluri, A. Raymond, T. Samikkannu, M.P. Nair, Enhanced blood-brain barrier transmigration using a novel transferrin embedded fluorescent magneto-liposome nanoformulation. Nanotechnology 25(5), 055101 (2014). doi:10.1088/0957-4484/25/5/055101
M. Kumar, G. Singh, V. Arora, S. Mewar, U. Sharma, N.R. Jagannathan, S. Sapra, A.K. Dinda, S. Kharbanda, H. Singh, Cellular interaction of folic acid conjugated superparamagnetic iron oxide nanoparticles and its use as contrast agent for targeted magnetic imaging of tumor cells. Int. J. Nanomedicine 7, 3503–3516 (2012)
M. Arsianti, M. Lim, C.P. Marquis, R. Amal, Assembly of polyethylenimine-based magnetic iron oxide vectors: insights into gene delivery. Langmuir 26(10), 7314–7326 (2010). doi:10.1021/la9041919
R. Namgung, K. Singha, M.K. Yu, S. Jon, Y.S. Kim, Y. Ahn, I.K. Park, W.J. Kim, Hybrid superparamagnetic iron oxide nanoparticle-branched polyethylenimine magnetoplexes for gene transfection of vascular endothelial cells. Biomaterials 31(14), 4204–4213 (2010). doi:10.1016/j.biomaterials.2010.01.123
S. Jiang, A.A. Eltoukhy, K.T. Love, R. Langer, D.G. Anderson, Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Lett. 13(3), 1059–1064 (2013). doi:10.1021/nl304287a
S.J. Soenen, S.C. De Smedt, K. Braeckmans, Limitations and caveats of magnetic cell labeling using transfection agent complexed iron oxide nanoparticles. Contrast Media Mol. Imaging 7(2), 140–152 (2012). doi:10.1002/cmmi.472
M. Babic, D. Horák, M. Trchová, P. Jendelová, K. Glogarová, P. Lesný, V. Herynek, M. Hájek, E. Syková, Poly(l-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjug. Chem. 19(3), 740–750 (2008). doi:10.1021/bc700410z
X. Wang, Q. Tu, B. Zhao, Y. An, J.C. Wang, W. Liu, M.S. Yuan, S.M. Ahmed, J. Xu, R. Liu, Y. Zhang, J. Wang, Effects of poly(l-lysine)-modified Fe3O4 nanoparticles on endogenous reactive oxygen species in cancer stem cells. Biomaterials 34(4), 1155–1169 (2013). doi:10.1016/j.biomaterials.2012.10.063
S. Shanehsazzadeh, M.A. Oghabian, B.J. Allen, M. Amanlou, A. Masoudi, F.J. Daha, Evaluating the effect of ultrasmall superparamagnetic iron oxide nanoparticles for a long-term magnetic cell labeling. J. Med. Phys. 38(1), 34–40 (2013). doi:10.4103/0971-6203.106603
Z. Wang, A. Cuschieri, Tumour cell labelling by magnetic nanoparticles with determination of intracellular iron content and spatial distribution of the intracellular iron. Int. J. Mol. Sci. 14(5), 9111–9125 (2013). doi:10.3390/ijms14059111
D. Mazia, G. Schatten, W. Sale, Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J. Cell Biol. 66(1), 198–200 (1975). doi:10.1083/jcb.66.1.198
M. Watanabe, M. Yoneda, A. Morohashi, Y. Hori, D. Okamoto et al., Effects of Fe3O4 magnetic nanoparticles on A549 cells. Int. J. Mol. Sci. 14(8), 15546–15560 (2013). doi:10.3390/ijms140815546
A. Akbarzadeh, M. Samiei, S.W. Joo, M. Anzaby, Y. Hanifehpour, H.T. Nasrabadi, S. Davaran, Synthesis, characterization and in vitro studies of doxorubicin-loaded magnetic nanoparticles grafted to smart copolymers on A549 lung cancer cell line. J. Nanobiotechnology 10, 46 (2012). doi:10.1186/1477-3155-10-46
S. Qu, H. Yang, D. Ren, S. Kan, G. Zou, D. Li, M. Li, Magnetite nanoparticles prepared by precipitation from partially reduced ferric chloride aqueous solutions. J. Colloid Interface Sci. 215(1), 190–192 (1999). doi:10.1006/jcis.1999.6185
Y.K. Sun, M. Ma, Y. Zhang, N. Gu, Synthesis of nanometer-size maghemite particles from magnetite. Colloids Surf. A. Physicochem Eng. Asp. 245(1–3), 15–19 (2004). doi:10.1016/j.colsurfa.2004.05.009
X. Wang, F. Wei, A. Liu, L. Wang, J.C. Wang, L. Ren, W. Liu, Q. Tu, L. Li, J. Wang, Cancer stem cell labeling using poly(l-lysine)-modified iron oxide nanoparticles. Biomaterials 33(14), 3719–3732 (2012). doi:10.1016/j.biomaterials.2012.01.058
B.W. Kristensen, H. Noer, J.B. Gramsbergen, J. Zimmer, J. Noraberg, Colchicine induces apoptosis in organotypic hippocampal slice cultures. Brain Res. 964(2), 264–278 (2003). doi:10.1016/S0006-8993(02)04080-5
X. Huang, L. Li, Q. Tu, J. Wang, W. Liu, X. Wang, L. Ren, J. Wang, On-chip cell migration assay for quantifying the effect of ethanol on MCF-7 human breast cancer cells. Microfluid. Nanofluidics 10(6), 1333–1341 (2011). doi:10.1007/s10404-011-0766-9
A.S. Arbab, G.T. Yocum, H. Kalish, E.K. Jordan, S.A. Anderson, A.Y. Khakoo, E.J. Read, J.A. Frank, Efficient magnetic cell labeling with protamine sulfate complexed to ferum-oxides for cellular MRI. Blood 104(4), 1217–1223 (2004). doi:10.1182/blood-2004-02-0655
D. Horák, M. Babič, P. Jendelová, V. Herynek, M. Trchová, Z. Pientka, E. Pollert, M. Hájek, E. Syková, d-mannose-modified iron oxide nanoparticles for stem cell labeling. Bioconjug. Chem. 18(3), 635–644 (2007). doi:10.1021/bc060186c
J. Wang, X. Wang, L. Ren, Q. Wang, L. Li et al., Conjugation of biomolecules with magnetic protein microspheres for the assay of early biomarkers associated with acute myocardial infarction. Anal. Chem. 81(15), 6210–6217 (2009). doi:10.1021/ac9007418
M. Neri, C. Maderna, C. Cavazzin, V. Deidda-Vigoriti, L.S. Politi et al., Efficient in vitro labeling of human neural precursor cells with superparamagnetic iron oxide particles: relevance for in vivo cell tracking. Stem Cells 26(2), 505–516 (2008). doi:10.1634/stemcells.2007-0251
S. Naqvi, M. Samim, M. Abdin, F.J. Ahmed, A. Maitra, C. Prashant, A.K. Dinda, Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomedicine 5, 983–989 (2010). doi:10.2147/IJN.S13244
S.J. Soenen, E. Illyes, D. Vercauteren, K. Braeckmans, Z. Majer, S.C. De Smedt, M. De Cuyper, The role of nanoparticle concentration-dependent induction of cellular stress in the internalization of non-toxic cationic magnetoliposomes. Biomaterials 30(36), 6803–6813 (2009). doi:10.1016/j.biomaterials.2009.08.050
S.J. Soenen, U. Himmelreich, N. Nuytten, M. De Cuyper, Cytotoxic effects of iron oxide nanoparticles and implications for safety in cell labeling. Biomaterials 32(1), 195–205 (2011). doi:10.1016/j.biomaterials.2010.08.075
A.H. Koyama, A. Adachi, Induction of apoptosis by herpes simplex virus type 1. J. Gen. Virol. 78(Pt 11), 2909–2912 (1997)
A.H. Koyama, Y. Miwa, Suppression of apoptotic DNA fragmentation in herpes simplex virus type 1-infected cells. J. Virol. 71(3), 2567–2571 (1997)
S. Elmore, Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35, 495–516 (2007). doi:10.1080/01926230701320337
S.R. Denmeade, J.T. Isaacs, Programmed cell death (apoptosis) and cancer chemotherapy. Cancer Control 3(4), 303–309 (1996)
K.F. Jorgenson, U. Varshney, J.H. van de Sande, Interaction of Hoechst 33258 with repeating synthetic DNA polymers and natural DNA. J. Biomol. Struct. Dyn. 5(5), 1005–1023 (1988). doi:10.1080/07391102.1988.10506446
S.Y. Breusegem, R.M. Clegg, F.G. Loontiens, Base-sequence specificity of Hoechst 33258 and DAPI binding to five (A/T)4 DNA sites with kinetic evidence for more than one high-affinity Hoechst 33258-AATT complex. J. Mol. Biol. 315(5), 1049–1061 (2002). doi:10.1006/jmbi.2001.5301