Electrochemical Aptasensor Based on Prussian Blue-Chitosan-Glutaraldehyde for the Sensitive Determination of Tetracycline
Corresponding Author: Xia Sun
Nano-Micro Letters,
Vol. 6 No. 2 (2014), Article Number: 143-152
Abstract
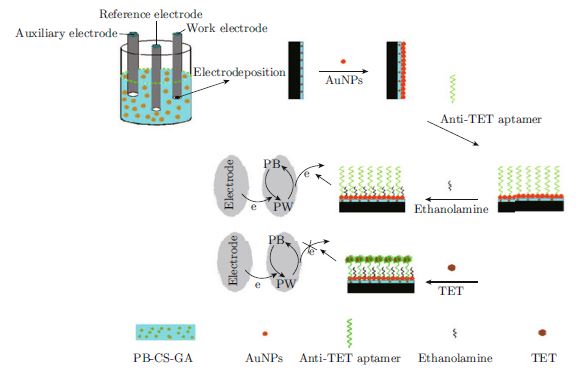
In this paper, a novel and sensitive electrochemical aptasensor for detecting tetracycline (TET) with prussian blue (PB) as the label-free signal was fabricated. A PB-chitosan-glutaraldehyde (PB-CS-GA) system acting as the signal indicator was developed to improve the sensitivity of the electrochemical aptasensor. Firstly, the PB-CS-GA was fixed onto the glass carbon electrode surface. Then, colloidal gold nanoparticles (AuNPs) were droped onto the electrode to immobilize the anti-TET aptamer for preparation of the aptasensor. The stepwise assembly process of the aptasensor was characterized by cyclic voltammetry (C-V) and scanning electron microscope (SEM). The target TET captured onto the electrode induced the current response of the electrode due to the non-conducting biomoleculars. Under the optimum operating conditions, the response of differential pulse voltammetry (DPV) was used for detecting the concentration of TET. The proposed aptasensor showed a high sensitivity and a wide linear range of 10−9 ∼ 10−5 M and 10−5 ∼ 10−2 M with the correlation coefficients of 0.994 and 0.992, respectively. The detection limit was 3.2×10−10 M (RSD 4.12%). Due to its rapidity, sensitivity and low cost, the proposed aptasensor could be used as a pre-scanning method in TET determination for the analysis of livestock products.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- K. Kishida, “Simplified extraction of tetracycline antibiotics from milk using a centrifugal ultrafiltration device”, Food Chem. 126(2), 687–690 (2011). http://dx.doi.org/10.1016/j.foodchem.2010.11.021
- D. E. Brodersen, W. M. Jr. Clemons, A. P. Carter, R. J. Morgan-Warren, B. T. Wimberly and V. Ramakrishnan, “The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit”, Cell 103(7), 1143–1154 (2000). http://dx.doi.org/10.1016/S0092-8674(00)00216-6
- F. K. Muriuki, “Tetracycline residue levels in cattle meat from Nairobi salughter house in Kenya”, J. Vet. Sci. 2(2), 97–101 (2001).
- N. Vragovic, D. Bazulic and B. Njari, “Risk assessment of streptomycin and tetracycline residues in meat and milk on Croatian market”, Food Chem. 49(2), 352–355 (2011). http://dx.doi.org/10.1016/j.fct.2010.11.006
- M. Kuhne, S. Wegmann, A. Kobe and R. Fries, “Tetracycline residues in bones of slaughtered animals”, Food Control. 11(3). 175–180 (2000). http://dx.doi.org/10.1016/S0956-7135(99)00092-4
- G. T. Peres, S. Rath and F. G. Reyes, “A HPLC with fluorescence detection method for the determination of tetracyclines residues and evaluation of their stability in honey”, Food Control. 21(5), 620–625 (2010). http://dx.doi.org/10.1016/j.foodcont.2009.09.006
- J. G. Salisbury, T. J. Nicholls, A. M. Lammerding, J. Turnidge, M. J. Nunn, “A risk analysis framework for the long-term management of antibiotic resistance in food-producing animals”. Int. J. Antimicrob. Agents 20(3), 153–164 (2002). http://dx.doi.org/10.1016/S0924-8579(02)00169-3
- Commission Regulation 508/1999/EC, 1999.
- Japanese Ministry of Health Welfare and Labor, 2008.
- US Food and Drug Administration, 1975.
- J. Kurittu, S. Lönnberg, M. Virta and M. Karp, “A group-specific microbiological test for the detection of tetracycline residues in raw milk”. J. Agric. Food. Chem. 48(8), 3372–3377 (2000). http://dx.doi.org/10.1021/jf9911794
- J. W. Fritz and Y. Zuo, “Simultaneous determination of tetracycline, oxytetracycline, and 4-epitetracycline in milk by high-performance liquid chromatography”, Food Chem. 105(3), 1297–1301 (2007). http://dx.doi.org/10.1016/j.foodchem.2007.03.047
- K. Ng and S. W. Linder, “HPLC separation of tetracycline analogues: comparison study of laser-based polarimetric detection with UV detection”, J. Chromatogr Sci. 41(9), 460–466 (2003). http://dx.doi.org/10.1093/chromsci/41.9.460
- A. C. Martel, S. Zeggane, P. Drajnudel, J. P. Faucon and M. Aubert, “Tetracycline residues in honey after hive treatment”, Food Addit. Contam. 23(3), 265–273 (2006). http://dx.doi.org/10.1080/02652030500469048
- P. Kowalski, “Capillary electrophoretic method for the simultaneous determination of tetracycline residues in fish samples”, J. Pharm. Biomed. 47(3), 487–493 (2008). http://dx.doi.org/10.1016/j.jpba.2008.01.036
- B. Y. Deng, Q. X. Xu, H. Lu, L. Ye and Y. Z. Wang, “Pharmacokinetics and residues of tetracycline in crucian carp muscle using capillary electrophoresis on-line coupled with electrochemiluminescence detection”, Food Chem. 134(4), 2350–2354 (2012). http://dx.doi.org/10.1016/j.foodchem.2012.03.117
- L. M. Shen, M. L. Chen and X. W. Chen, “A novel flow-through fluorescence optosensor for the sensitive determination of tetracycline”, Talanta 85(3), 1285–1290 (2011). http://dx.doi.org/10.1016/j.talanta.2011.06.006
- N. Rodríguez, B. D. Real, M. Cruz Ortiz, L. A. Sarabia, A. Herrero, “Usefulness of parallel factor analysis to handle the matrix effect in the fluorescence determination of tetracycline in whey milk”, Anal. Chim. Acta 632(1), 42–51 (2009). http://dx.doi.org/10.1016/j.aca.2008.10.051
- H. Oka, Y. Ito, Y. Ikai, T. Kagami and K. Harada, “Mass spectrometric analysis of tetracycline antibiotics in foods”, J. Chromatogr. A. 812(1–2), 309–319 (1998). http://dx.doi.org/10.1016/S0021-9673(97)01278-8
- M. E. Dasenaki and N. S. Thomaidis, “Multi-residue determination of seventeen sulfonamides and five tetracyclines in fish tissue using a multi-stage LC-ESIMS/MS approach based on advanced mass spectrometric techniques”, Anal. Chim. Acta 672(1–2), 93–102 (2010). http://dx.doi.org/10.1016/j.aca.2010.04.034
- F. Conzuelo, M. Gamella, S. Campuzano, A. Julio Reviejo and J. M. Pingarrón, “Disposable amperometric magneto-immunosensor for direct detection of tetracyclines antibiotics residues in milk”, Anal. Chim. Acta 737(6), 29–36 (2012). http://dx.doi.org/10.1016/j.aca.2012.05.051
- M. Jeon, J. Kim, K. J. Paeng, S. W. Park and I. R. Paeng, “Biotin-avidin mediated competitive enzymelinked immunosorbent assay to detect residues of tetracyclines in milk”, Microchem. J. 88(1), 26–31 (2008). http://dx.doi.org/10.1016/j.microc.2007.09.001
- L. Zhou, D. J. Li, L. Gai, J. P. Wang and Y. B. Li, “Electrochemical aptasensor for the detection of tetracycline with multi-walled carbon nanotubes amplification”, Sens. Actuators, B: Chem. 162(1), 201–208 (2012). http://dx.doi.org/10.1016/j.snb.2011.12.067
- A. D. Ellington and J. W. Szostak, “In vitro selection of RNA molecules that bind specific ligands”, Nature 346, 818–822 (1990). http://dx.doi.org/10.1038/346818a0
- C. Tuerk and L. Gold, “Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase”, Science 249(4968), 505–510 (1990). http://dx.doi.org/10.1126/science.2200121
- C. Pestourie, B. Tavitian and F. Duconge, “Aptamers against extracellular targets for in vivo applications”, Biochimie 87(9–10), 921–930 (2005). http://dx.doi.org/10.1016/j.biochi.2005.04.013
- C. A. Savran, S. M. Knudsen, A. D. Ellington and S. R. Manalis, “Micromechanical detection of proteins using aptamer-based receptor molecules”. Anal. Chem. 76(11), 3194–3198 (2004). http://dx.doi.org/10.1021/ac049859f
- Y. J. Kim, Y. S. Kim, J. H. Niazi and M. B. Gu, “Electrochemical aptasensor for tetracycline detection”, Bioprocess. Biosyst. Eng. 33(1), 31–37 (2010). http://dx.doi.org/10.1007/s00449-009-0371-4
- K. Kerman, M. Saito, S. Yamamura, Y. Takamura and E. Tamiya, “Nanomaterial-based electrochemical biosensors for medical applications”, Trends Anal. Chem. 27(7), 585–592 (2008). http://dx.doi.org/10.1016/j.trac.2008.05.004
- Y. T. Shi, R. Yuan, Y. Q. Chai, M. Y. Tang and X. L. He, “Amplification of antigen-antibody interactions via back-filling of HRP on the layer-bylayer self-assembling of thionine and gold nanoparticles films on Titania nanoparticles/gold nanoparticlescoated Au electrode”, J. Electroanal. Chem. 604(1), 9–16 (2007). http://dx.doi.org/10.1016/j.jelechem.2007.02.027
- S. Y. Xu and X. Z. Han, “A novel method to construct a third-generation biosensor:self-assembling gold nanoparticles on thiol-functionalized poly(styrene-co -acrylic acid) nanospheres”, Biosens. Bioelectron. 19(9), 1117–1120 (2004). http://dx.doi.org/10.1016/j.bios.2003.09.007
- S. Q. Liu, D. Leech and H. X. Ju, “Application of colloidal gold in protein immobilization, electron transfer, and biosensing”, Anal. Lett. 36(1), 1–19 (2003). http://dx.doi.org/10.1081/al-120017740
- P. Sorlier, A. Denuzière, C. Viton and A. Domard, “Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan”, Biomacromolecules 2(3), 765–772 (2001). http://dx.doi.org/10.1021/bm015531+
- J. H. Niazi, S. J. Lee and M. B. Gu, “Single-stranded, DNA aptamers specific for antibiotics tetracyclines”, Bioorg. Med. Chem. 16(15), 7245–7253 (2008). http://dx.doi.org/10.1016/j.bmc.2008.06.033
- K.C. Grabar, R.G. Freeman, M. B. Hommer and M.J. Natan, “Preparation and characterization of Au colloid monolayers”, Anal. Chem. 67(4), 735–743 (1995). http://dx.doi.org/10.1021/ac00100a008
- C. Cao, J. P. Kim, B. W. Kim, H. Chae, H. C. Yoon, S. S. Yang and S. J. Sim, “A strategy for sensitivity and specificity enhancements in prostate specific antigen-a1-antichymotrypsin detection based on surface plasmon resonance”, Biosens. Bioelectron. 21(11), 2106–2113 (2006). http://dx.doi.org/10.1016/j.bios.2005.10.014
- Y. Wang, W. H. Liu, K. M. Wang, G. L. Shen and R. Q. Yu. “Fluorescence optical fiber sensor for tetracycline”, Talanta. 47(1), 33–42 (1998). http://dx.doi.org/10.1016/S0039-9140(98)00049-6
- H. Zhao, H. T. Wang, X. Quan and F. Tan, “Amperometric Sensor for Tetracycline Determination Based on Molecularly Imprinted Technique”, Procedia Environmental Sciences 18, 249–257 (2013). http://dx.doi.org/10.1016/j.proenv.2013.04.032
- P. Su, N. Liu, M. X. Zhu, B. A. Ning, M. Liu, Z. H. Yang, X. J. Pan and Z. X. Gao, “Simultaneous detection of five antibiotics in milk by high-throughput suspension array technology”. Talanta 85(2), 1160–1165 (2011). http://dx.doi.org/10.1016/j.talanta.2011.05.040
- E. Karageorgou, M. Armeni, I. Moschou, and V. Samanidou, “Ultrasound-assisted dispersive extraction for the high pressure liquid chromatographic determination of tetracyclines residues in milk with diode array detection”. Food Chem. 150(1), 328–334 (2014). http://dx.doi.org/10.1016/j.foodchem.2013.11.008
References
K. Kishida, “Simplified extraction of tetracycline antibiotics from milk using a centrifugal ultrafiltration device”, Food Chem. 126(2), 687–690 (2011). http://dx.doi.org/10.1016/j.foodchem.2010.11.021
D. E. Brodersen, W. M. Jr. Clemons, A. P. Carter, R. J. Morgan-Warren, B. T. Wimberly and V. Ramakrishnan, “The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit”, Cell 103(7), 1143–1154 (2000). http://dx.doi.org/10.1016/S0092-8674(00)00216-6
F. K. Muriuki, “Tetracycline residue levels in cattle meat from Nairobi salughter house in Kenya”, J. Vet. Sci. 2(2), 97–101 (2001).
N. Vragovic, D. Bazulic and B. Njari, “Risk assessment of streptomycin and tetracycline residues in meat and milk on Croatian market”, Food Chem. 49(2), 352–355 (2011). http://dx.doi.org/10.1016/j.fct.2010.11.006
M. Kuhne, S. Wegmann, A. Kobe and R. Fries, “Tetracycline residues in bones of slaughtered animals”, Food Control. 11(3). 175–180 (2000). http://dx.doi.org/10.1016/S0956-7135(99)00092-4
G. T. Peres, S. Rath and F. G. Reyes, “A HPLC with fluorescence detection method for the determination of tetracyclines residues and evaluation of their stability in honey”, Food Control. 21(5), 620–625 (2010). http://dx.doi.org/10.1016/j.foodcont.2009.09.006
J. G. Salisbury, T. J. Nicholls, A. M. Lammerding, J. Turnidge, M. J. Nunn, “A risk analysis framework for the long-term management of antibiotic resistance in food-producing animals”. Int. J. Antimicrob. Agents 20(3), 153–164 (2002). http://dx.doi.org/10.1016/S0924-8579(02)00169-3
Commission Regulation 508/1999/EC, 1999.
Japanese Ministry of Health Welfare and Labor, 2008.
US Food and Drug Administration, 1975.
J. Kurittu, S. Lönnberg, M. Virta and M. Karp, “A group-specific microbiological test for the detection of tetracycline residues in raw milk”. J. Agric. Food. Chem. 48(8), 3372–3377 (2000). http://dx.doi.org/10.1021/jf9911794
J. W. Fritz and Y. Zuo, “Simultaneous determination of tetracycline, oxytetracycline, and 4-epitetracycline in milk by high-performance liquid chromatography”, Food Chem. 105(3), 1297–1301 (2007). http://dx.doi.org/10.1016/j.foodchem.2007.03.047
K. Ng and S. W. Linder, “HPLC separation of tetracycline analogues: comparison study of laser-based polarimetric detection with UV detection”, J. Chromatogr Sci. 41(9), 460–466 (2003). http://dx.doi.org/10.1093/chromsci/41.9.460
A. C. Martel, S. Zeggane, P. Drajnudel, J. P. Faucon and M. Aubert, “Tetracycline residues in honey after hive treatment”, Food Addit. Contam. 23(3), 265–273 (2006). http://dx.doi.org/10.1080/02652030500469048
P. Kowalski, “Capillary electrophoretic method for the simultaneous determination of tetracycline residues in fish samples”, J. Pharm. Biomed. 47(3), 487–493 (2008). http://dx.doi.org/10.1016/j.jpba.2008.01.036
B. Y. Deng, Q. X. Xu, H. Lu, L. Ye and Y. Z. Wang, “Pharmacokinetics and residues of tetracycline in crucian carp muscle using capillary electrophoresis on-line coupled with electrochemiluminescence detection”, Food Chem. 134(4), 2350–2354 (2012). http://dx.doi.org/10.1016/j.foodchem.2012.03.117
L. M. Shen, M. L. Chen and X. W. Chen, “A novel flow-through fluorescence optosensor for the sensitive determination of tetracycline”, Talanta 85(3), 1285–1290 (2011). http://dx.doi.org/10.1016/j.talanta.2011.06.006
N. Rodríguez, B. D. Real, M. Cruz Ortiz, L. A. Sarabia, A. Herrero, “Usefulness of parallel factor analysis to handle the matrix effect in the fluorescence determination of tetracycline in whey milk”, Anal. Chim. Acta 632(1), 42–51 (2009). http://dx.doi.org/10.1016/j.aca.2008.10.051
H. Oka, Y. Ito, Y. Ikai, T. Kagami and K. Harada, “Mass spectrometric analysis of tetracycline antibiotics in foods”, J. Chromatogr. A. 812(1–2), 309–319 (1998). http://dx.doi.org/10.1016/S0021-9673(97)01278-8
M. E. Dasenaki and N. S. Thomaidis, “Multi-residue determination of seventeen sulfonamides and five tetracyclines in fish tissue using a multi-stage LC-ESIMS/MS approach based on advanced mass spectrometric techniques”, Anal. Chim. Acta 672(1–2), 93–102 (2010). http://dx.doi.org/10.1016/j.aca.2010.04.034
F. Conzuelo, M. Gamella, S. Campuzano, A. Julio Reviejo and J. M. Pingarrón, “Disposable amperometric magneto-immunosensor for direct detection of tetracyclines antibiotics residues in milk”, Anal. Chim. Acta 737(6), 29–36 (2012). http://dx.doi.org/10.1016/j.aca.2012.05.051
M. Jeon, J. Kim, K. J. Paeng, S. W. Park and I. R. Paeng, “Biotin-avidin mediated competitive enzymelinked immunosorbent assay to detect residues of tetracyclines in milk”, Microchem. J. 88(1), 26–31 (2008). http://dx.doi.org/10.1016/j.microc.2007.09.001
L. Zhou, D. J. Li, L. Gai, J. P. Wang and Y. B. Li, “Electrochemical aptasensor for the detection of tetracycline with multi-walled carbon nanotubes amplification”, Sens. Actuators, B: Chem. 162(1), 201–208 (2012). http://dx.doi.org/10.1016/j.snb.2011.12.067
A. D. Ellington and J. W. Szostak, “In vitro selection of RNA molecules that bind specific ligands”, Nature 346, 818–822 (1990). http://dx.doi.org/10.1038/346818a0
C. Tuerk and L. Gold, “Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase”, Science 249(4968), 505–510 (1990). http://dx.doi.org/10.1126/science.2200121
C. Pestourie, B. Tavitian and F. Duconge, “Aptamers against extracellular targets for in vivo applications”, Biochimie 87(9–10), 921–930 (2005). http://dx.doi.org/10.1016/j.biochi.2005.04.013
C. A. Savran, S. M. Knudsen, A. D. Ellington and S. R. Manalis, “Micromechanical detection of proteins using aptamer-based receptor molecules”. Anal. Chem. 76(11), 3194–3198 (2004). http://dx.doi.org/10.1021/ac049859f
Y. J. Kim, Y. S. Kim, J. H. Niazi and M. B. Gu, “Electrochemical aptasensor for tetracycline detection”, Bioprocess. Biosyst. Eng. 33(1), 31–37 (2010). http://dx.doi.org/10.1007/s00449-009-0371-4
K. Kerman, M. Saito, S. Yamamura, Y. Takamura and E. Tamiya, “Nanomaterial-based electrochemical biosensors for medical applications”, Trends Anal. Chem. 27(7), 585–592 (2008). http://dx.doi.org/10.1016/j.trac.2008.05.004
Y. T. Shi, R. Yuan, Y. Q. Chai, M. Y. Tang and X. L. He, “Amplification of antigen-antibody interactions via back-filling of HRP on the layer-bylayer self-assembling of thionine and gold nanoparticles films on Titania nanoparticles/gold nanoparticlescoated Au electrode”, J. Electroanal. Chem. 604(1), 9–16 (2007). http://dx.doi.org/10.1016/j.jelechem.2007.02.027
S. Y. Xu and X. Z. Han, “A novel method to construct a third-generation biosensor:self-assembling gold nanoparticles on thiol-functionalized poly(styrene-co -acrylic acid) nanospheres”, Biosens. Bioelectron. 19(9), 1117–1120 (2004). http://dx.doi.org/10.1016/j.bios.2003.09.007
S. Q. Liu, D. Leech and H. X. Ju, “Application of colloidal gold in protein immobilization, electron transfer, and biosensing”, Anal. Lett. 36(1), 1–19 (2003). http://dx.doi.org/10.1081/al-120017740
P. Sorlier, A. Denuzière, C. Viton and A. Domard, “Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan”, Biomacromolecules 2(3), 765–772 (2001). http://dx.doi.org/10.1021/bm015531+
J. H. Niazi, S. J. Lee and M. B. Gu, “Single-stranded, DNA aptamers specific for antibiotics tetracyclines”, Bioorg. Med. Chem. 16(15), 7245–7253 (2008). http://dx.doi.org/10.1016/j.bmc.2008.06.033
K.C. Grabar, R.G. Freeman, M. B. Hommer and M.J. Natan, “Preparation and characterization of Au colloid monolayers”, Anal. Chem. 67(4), 735–743 (1995). http://dx.doi.org/10.1021/ac00100a008
C. Cao, J. P. Kim, B. W. Kim, H. Chae, H. C. Yoon, S. S. Yang and S. J. Sim, “A strategy for sensitivity and specificity enhancements in prostate specific antigen-a1-antichymotrypsin detection based on surface plasmon resonance”, Biosens. Bioelectron. 21(11), 2106–2113 (2006). http://dx.doi.org/10.1016/j.bios.2005.10.014
Y. Wang, W. H. Liu, K. M. Wang, G. L. Shen and R. Q. Yu. “Fluorescence optical fiber sensor for tetracycline”, Talanta. 47(1), 33–42 (1998). http://dx.doi.org/10.1016/S0039-9140(98)00049-6
H. Zhao, H. T. Wang, X. Quan and F. Tan, “Amperometric Sensor for Tetracycline Determination Based on Molecularly Imprinted Technique”, Procedia Environmental Sciences 18, 249–257 (2013). http://dx.doi.org/10.1016/j.proenv.2013.04.032
P. Su, N. Liu, M. X. Zhu, B. A. Ning, M. Liu, Z. H. Yang, X. J. Pan and Z. X. Gao, “Simultaneous detection of five antibiotics in milk by high-throughput suspension array technology”. Talanta 85(2), 1160–1165 (2011). http://dx.doi.org/10.1016/j.talanta.2011.05.040
E. Karageorgou, M. Armeni, I. Moschou, and V. Samanidou, “Ultrasound-assisted dispersive extraction for the high pressure liquid chromatographic determination of tetracyclines residues in milk with diode array detection”. Food Chem. 150(1), 328–334 (2014). http://dx.doi.org/10.1016/j.foodchem.2013.11.008