Exact Geometric Relationships, Symmetry Breaking and Structural Stability for Single-Walled Carbon Nanotubes
Corresponding Author: Tong Zhang
Nano-Micro Letters,
Vol. 3 No. 4 (2011), Article Number: 228-235
Abstract
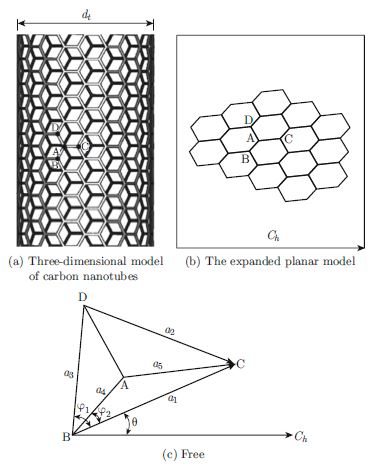
We pioneered a study about how the geometric relationship of single-walled carbon nanotubes (SWCNT) is influenced by curvature factor and non-planar geometry factor in cylindrical coordinate system based on the assumption of complete symmetry. The bond length and angle of every carbon-carbon bonds are determined by using the principle of the minimum energy. The results of the paper include:(1) From the calculation result, the symmetry breaking appears for chiral carbon nanotubes, while the part symmetry appears for achiral carbon nanotubes with increasing curvature. (2) The synergistic effect of bond lengths and bond angles is first found. (3) We conclude that the influence of non-planar geometry factor can be completely ignored on bond lengths and bond angles when the curvature parameter has been included in the model. (4) The two fractal dimensions are given from the nanoscale to the macroscale for zigzag topology and armchair topology respectively. Fractal dimensions of SWCNT show special characteristics, varying with the length of SWCNT until the lengths approach infinity. The close and inevitable correlations among curvature, symmetry breaking and stability of SWCNTs can be summed up as: the increase of curvature causes symmetry breaking, and such symmetry breaking will further reduce the structural stability.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- B. J. Cox and J. M. Hill, Carbon 45, 1453 (2007). http://dx.doi.org/10.1016/j.carbon.2007.03.028
- R. Setton, Carbon 33, 135 (1995). http://dx.doi.org/10.1016/0008-6223(94)00117-I
- K. F. Richard, B. J. Cox and J. M. Hill J. Math. Chem. 47, 569 (2010). http://dx.doi.org/10.1007/s10910-009-9586-5
- D. Baowan, B. J. Cox and J. M. Hill, J. Math. Chem. 44, 515 (2008). http://dx.doi.org/10.1007/s10910-007-9325-8
- V. K. Jindal and A. N. Imtani, Comp. Mat. Sci. 44, 156 (2008). http://dx.doi.org/10.1016/j.commatsci.2008.01.020
- H. Jiang, P. Zhang, B. Liu, Y. Huang, P. h. Geubelle and H. Gao, et al, Comp. Mat. Sci. 28, 429 (2003). http://dx.doi.org/10.1016/j.commatsci.2003.08.004
- Z. Y. Zhang and Z. Y. Yang, “MATLAB Tutorial”, Press of Beijing University of Aeronautics and Astronautics (2002).
- Kenneth Falconer, 2rd ed. Chichester, John Wiley & Sons Ltd (2003).
- T. Zhang, G. W. Tang, Y. J. Yin, X. Feng and Z. Lu, J. Nanosci. Nanotech. 7, 1 (2010). http://dx.doi.org/10.1504/IJNT.2010.029546
References
B. J. Cox and J. M. Hill, Carbon 45, 1453 (2007). http://dx.doi.org/10.1016/j.carbon.2007.03.028
R. Setton, Carbon 33, 135 (1995). http://dx.doi.org/10.1016/0008-6223(94)00117-I
K. F. Richard, B. J. Cox and J. M. Hill J. Math. Chem. 47, 569 (2010). http://dx.doi.org/10.1007/s10910-009-9586-5
D. Baowan, B. J. Cox and J. M. Hill, J. Math. Chem. 44, 515 (2008). http://dx.doi.org/10.1007/s10910-007-9325-8
V. K. Jindal and A. N. Imtani, Comp. Mat. Sci. 44, 156 (2008). http://dx.doi.org/10.1016/j.commatsci.2008.01.020
H. Jiang, P. Zhang, B. Liu, Y. Huang, P. h. Geubelle and H. Gao, et al, Comp. Mat. Sci. 28, 429 (2003). http://dx.doi.org/10.1016/j.commatsci.2003.08.004
Z. Y. Zhang and Z. Y. Yang, “MATLAB Tutorial”, Press of Beijing University of Aeronautics and Astronautics (2002).
Kenneth Falconer, 2rd ed. Chichester, John Wiley & Sons Ltd (2003).
T. Zhang, G. W. Tang, Y. J. Yin, X. Feng and Z. Lu, J. Nanosci. Nanotech. 7, 1 (2010). http://dx.doi.org/10.1504/IJNT.2010.029546