Thermodynamic and Kinetic Analysis of Lowtemperature Thermal Reduction of Graphene Oxide
Corresponding Author: Litao Sun
Nano-Micro Letters,
Vol. 3 No. 1 (2011), Article Number: 51-55
Abstract
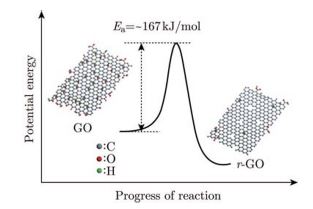
The thermodynamic state and kinetic process of low-temperature deoxygenation reaction of graphene oxide (GO) have been investigated for better understanding on the reduction mechanism by using Differential Scanning Calorimetry (DSC), Thermogravimetry-Mass Spectrometry (TG-MS), and X-ray Photo-electron Spectroscopy (XPS). It is found that the thermal reduction reaction of GO is exothermic with degassing of CO2, CO and H2O. Graphene is thermodynamically more stable than GO. The deoxygenation reaction of GO is kinetically controlled and the activation energy for GO is calculated to be 167kJ/mol (1.73 eV/atom).
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science 306, 666 (2004). http://dx.doi.org/10.1126/science.1102896
- S. Park and R. S. Ruoff, Nat. Nanotech. 4, 217 (2009). http://dx.doi.org/10.1038/nnano.2009.58
- X. S. Li, W. W. Cai, J. An, S. Kim, J. Nah, D. X. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. S. Ruoff, Science 324, 1312 (2009). http://dx.doi.org/10.1126/science.1171245
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev. 39, 228 (2010). http://dx.doi.org/10.1039/b917103g
- L. Kou, H. K. He and C. Gao, Nano-Micro Lett. 2, 177 (2010). http://dx.doi.org/10.5101/nml.v2i3. p177-183
- X. F. Gao, J. Jang and S. Nagase, J. Phys. Chem. C 114, 832 (2010). http://dx.doi.org/10.1021/jp909284g
- M. J. McAllister, J. L. Li, D. H. Adamson, H. C. Schniepp, A. A. Abdala, J. Liu, M. H. Alonso, D. L. Milius, R. Car, R. K. Prudhomme and I. A. Aksay, Chem. Mater. 19, 4396 (2007). http://dx.doi.org/10.1021/cm0630800
- Y. Zhu, M. D. Stoller, W. Cai, A. Velamakanni, R. D. Piner, D. Chen, and R. S. Ruoff, ACS Nano 4, 1227 (2010). http://dx.doi.org/10.1021/nn901689k
- W. F. Chen and L. F. Yan, Nanoscale 2, 559 (2010). http://dx.doi.org/10.1039/b9nr00191c
- S. Gilje, S. Dubin, A. Badakhshan, J. Farrar, S. A. Danczyk and R. B. Kaner, Adv. Mater. 22, 419 (2009). http://dx.doi.org/10.1002/adma.200901902
- L. J. Cote, R. Cruz-Silva and J. X. Huang, J. Am. Chem. Soc. 131, 11027 (2009). http://dx.doi.org/10.1021/ja902348k
- Y. L. Zhang, L. Guo, S. Wei, Y. Y. He, H. Xia, Q. D. Chen, H. B. Sun and F. S. Xiao, NanoToday 5, 15 (2010). http://dx.doi.org/10.1016/j.nantod.2009.12.009
- Y. Zhou, Q. L. Bao, L. A. L. Tang, Y. L. Zhong and K. P. Loh, Chem. Mater. 21, 2950 (2009). http://dx.doi.org/10.1021/cm9006603
- C. Nethravathi and M. Rajamathi, Carbon 46, 1994 (2008). http://dx.doi.org/10.1016/j.carbon.2008.08.013
- K. B. Yin, Y. D. Xia, C. Y. Chan, W. Q. Zhang, Q. J. Wang, X. N. Zhao, A. D. Li, Z. G. Liu, M. W. Bayes and K. W. Yee, Scripta Mater. 58, 65 (2008). http://dx.doi.org/10.1016/j.scriptamat.2007.08.028
- I. Jung, D. A. Field, N. J. Clark, Y. W. Zhu, D. X. Yang, R. D. Piner, S. Stankovich, D. A. Dikin, H. K. Geisler, C. A. Ventrice Jr and R. S. Ruoff, J. Phys. Chem. C 113, 18480 (2009). http://dx.doi.org/10.1021/jp904396j
- S. Deguchi, S. K. Ghosh, R. G. Alargova and K. Tsujii, J. Phys. Chem. B 110, 18358 (2006). http://dx.doi.org/10.1021/jp062045d
- A. Bagri, C. Mattevi, M. Acik, Y. J. Chabal, M. Chhowalla and V. B. Shenoy, Nat. Chem. 2, 581 (2010). http://dx.doi.org/10.1038/nchem.686
References
K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science 306, 666 (2004). http://dx.doi.org/10.1126/science.1102896
S. Park and R. S. Ruoff, Nat. Nanotech. 4, 217 (2009). http://dx.doi.org/10.1038/nnano.2009.58
X. S. Li, W. W. Cai, J. An, S. Kim, J. Nah, D. X. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. S. Ruoff, Science 324, 1312 (2009). http://dx.doi.org/10.1126/science.1171245
D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev. 39, 228 (2010). http://dx.doi.org/10.1039/b917103g
L. Kou, H. K. He and C. Gao, Nano-Micro Lett. 2, 177 (2010). http://dx.doi.org/10.5101/nml.v2i3. p177-183
X. F. Gao, J. Jang and S. Nagase, J. Phys. Chem. C 114, 832 (2010). http://dx.doi.org/10.1021/jp909284g
M. J. McAllister, J. L. Li, D. H. Adamson, H. C. Schniepp, A. A. Abdala, J. Liu, M. H. Alonso, D. L. Milius, R. Car, R. K. Prudhomme and I. A. Aksay, Chem. Mater. 19, 4396 (2007). http://dx.doi.org/10.1021/cm0630800
Y. Zhu, M. D. Stoller, W. Cai, A. Velamakanni, R. D. Piner, D. Chen, and R. S. Ruoff, ACS Nano 4, 1227 (2010). http://dx.doi.org/10.1021/nn901689k
W. F. Chen and L. F. Yan, Nanoscale 2, 559 (2010). http://dx.doi.org/10.1039/b9nr00191c
S. Gilje, S. Dubin, A. Badakhshan, J. Farrar, S. A. Danczyk and R. B. Kaner, Adv. Mater. 22, 419 (2009). http://dx.doi.org/10.1002/adma.200901902
L. J. Cote, R. Cruz-Silva and J. X. Huang, J. Am. Chem. Soc. 131, 11027 (2009). http://dx.doi.org/10.1021/ja902348k
Y. L. Zhang, L. Guo, S. Wei, Y. Y. He, H. Xia, Q. D. Chen, H. B. Sun and F. S. Xiao, NanoToday 5, 15 (2010). http://dx.doi.org/10.1016/j.nantod.2009.12.009
Y. Zhou, Q. L. Bao, L. A. L. Tang, Y. L. Zhong and K. P. Loh, Chem. Mater. 21, 2950 (2009). http://dx.doi.org/10.1021/cm9006603
C. Nethravathi and M. Rajamathi, Carbon 46, 1994 (2008). http://dx.doi.org/10.1016/j.carbon.2008.08.013
K. B. Yin, Y. D. Xia, C. Y. Chan, W. Q. Zhang, Q. J. Wang, X. N. Zhao, A. D. Li, Z. G. Liu, M. W. Bayes and K. W. Yee, Scripta Mater. 58, 65 (2008). http://dx.doi.org/10.1016/j.scriptamat.2007.08.028
I. Jung, D. A. Field, N. J. Clark, Y. W. Zhu, D. X. Yang, R. D. Piner, S. Stankovich, D. A. Dikin, H. K. Geisler, C. A. Ventrice Jr and R. S. Ruoff, J. Phys. Chem. C 113, 18480 (2009). http://dx.doi.org/10.1021/jp904396j
S. Deguchi, S. K. Ghosh, R. G. Alargova and K. Tsujii, J. Phys. Chem. B 110, 18358 (2006). http://dx.doi.org/10.1021/jp062045d
A. Bagri, C. Mattevi, M. Acik, Y. J. Chabal, M. Chhowalla and V. B. Shenoy, Nat. Chem. 2, 581 (2010). http://dx.doi.org/10.1038/nchem.686