Study of Cell Behaviors on Anodized TiO2 Nanotube Arrays with Coexisting Multi-Size Diameters
Corresponding Author: Xiaonong Zhang
Nano-Micro Letters,
Vol. 8 No. 1 (2016), Article Number: 61-69
Abstract
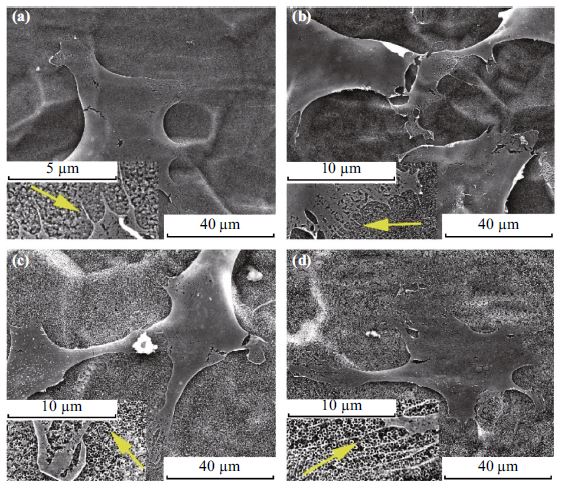
It has been revealed that the different morphologies of anodized TiO2 nanotubes, especially nanotube diameters, triggered different cell behaviors. However, the influence of TiO2 nanotubes with coexisting multi-size diameters on cell behaviors is seldom reported. In this work, coexisting four-diameter TiO2 nanotube samples, namely, one single substrate with the integration of four different nanotube diameters (60, 150, 250, and 350 nm), were prepared by repeated anodization. The boundaries between two different diameter regions show well-organized structure without obvious difference in height. The adhesion behaviors of MC3T3-E1 cells on the coexisting four-diameter TiO2 nanotube arrays were investigated. The results exhibit a significant difference of cell density between smaller diameters (60 and 150 nm) and larger diameters (250 and 350 nm) within 24 h incubation with the coexistence of different diameters, which is totally different from that on the single-diameter TiO2 nanotube arrays. The coexistence of four different diameters does not change greatly the cell morphologies compared with the single-diameter nanotubes. The findings in this work are expected to offer further understanding of the interaction between cells and materials.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- M. Ventre, F. Cause, P.A. Netti, Determinants of cell-material crosstalk at the interface: towards engineering of cell instructive materials. J. R. Soc. Interface 9, 2017–2032 (2012). doi:10.1098/rsif.2012.0308
- J.T. Parsons, A.R. Horwitz, M.A. Schwartz, Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11, 633–643 (2010). doi:10.1038/nrm2957
- T.W. Qin, Z.M. Yang, Z.Z. Wu, H.Q. Xie, J. Qin, S.X. Cai, Adhesion strength of human tenocytes to extracellular matrix component-modified poly(dl-lactide-co-glycolide) substrates. Biomaterials 26(33), 6635–6642 (2005). doi:10.1016/j.biomaterials.2005.04.023
- Z. Gao, S. Wang, H. Zhu, C.N. Su, G.L. Xu, X.J. Lian, Using selected uniform cells in round shape with a micropipette to measure cell adhesion strength on silk fibroin-based materials. Mater. Sci. Eng. C 28(8), 1227–1235 (2008). doi:10.1016/j.msec.2007.11.003
- K. Anselme, M. Bigerelle, Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomater. 1(2), 211–222 (2005). doi:10.1016/j.actbio.2004.11.009
- D. Regonini, C.R. Bowen, A. Jaroenworaluck, R. Stevens, A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 74(12), 377–406 (2013). doi:10.1016/j.mser.2013.10.001
- G.K. Mor, O.K. Varghese, M. Paulose, K. Shankar, C.A. Grimes, A review on highly ordered, vertically oriented TiO2 nanotube arrays: fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 90(14), 2011–2075 (2006). doi:10.1016/j.solmat.2006.04.007
- E. Gultepe, D. Nagesha, S. Sridhar, M. Amiji, Nanoporous inorganic membranes or coatings for sustained drug delivery in implantable devices. Adv. Drug Del. Rev. 62, 305–315 (2010). doi:10.1016/j.addr.2009.11.003
- A. Kodama, S. Bauer, A. Komatsu, H. Asoh, S. Ono, P. Schmuki, Bioactivation of titanium surfaces using coatings of TiO2 nanotubes rapidly pre-loaded with synthetic hydroxyapatite. Acta Biomater. 5(6), 2322–2330 (2009). doi:10.1016/j.actbio.2009.02.032
- S.P. Albu, A. Ghicov, S. Aldabergenova, P. Drechsel, D.J. LeClere, G.E. Thompson, J.M. Macak, P. Schmuki, Formation of double-walled TiO2 nanotubes and robust anatase membranes. Adv. Mater. 20(21), 4135–4139 (2008). doi:10.1002/adma.200801189
- H. Yin, H. Liu, W.Z. Shen, The large diameter and fast growth of self-organized TiO2 nanotube arrays achieved via electrochemical anodization. Nanotechnology 21, 035601 (2010). doi:10.1088/0957-4484/21/3/035601
- S. Oh, C. Daraio, L.H. Chen, T.R. Pisantic, R.R. Finones, S. Jin, Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J. Biomed. Mater. Res. A 78(1), 97–103 (2006). doi:10.1002/jbm.a.30722
- K. Das, S. Bose, A. Bandyopadhyay, TiO2 nanotubes on Ti: influence of nanoscale morphology on bone cell-materials interaction. J. Biomed. Mater. Res. A 90(1), 225–237 (2009). doi:10.1002/jbm.a.32088
- S. Oh, S. Jin, Titanium oxide nanotubes with controlled morphology for enhanced bone growth. Mater. Sci. Eng. C 26(8), 1301–1306 (2006). doi:10.1016/j.msec.2005.08.014
- K.S. Brammer, C.J. Frandsen, S. Jin, TiO2 nanotubes for bone regeneration. Trends Biotechnol. 30(6), 315–322 (2012). doi:10.1016/j.tibtech.2012.02.005
- J.H. Ni, C.J. Frandsen, K. Noh, G.W. Johnston, G. He, T.T. Tang, S. Jin, Fabrication of thin film TiO2 nanotube arrays on Co-28Cr-6Mo alloy by anodization. Mater. Sci. Eng. C 33, 1460–1466 (2013). doi:10.1016/j.msec.2012.12.068
- J. Park, S. Bauer, V.D. Mark, P. Schmuki, Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 7(6), 1686–1691 (2007). doi:10.1021/nl070678d
- J. Park, S. Bauer, K.A. Schlegel, F.W. Neukam, K. Von der Mark, P. Schmuki, TiO2 nanotube surfaces: 15 nm—an optimal length scale of surface topography for cell adhesion and differentiation. Small 5(6), 666–671 (2009). doi:10.1002/smll.200801476
- K.S. Brammer, S. Oh, C.J. Cobb, L.M. Bjursten, H.V.D. Heyde, S. Jin, Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 5(8), 3215–3223 (2009). doi:10.1016/j.actbio.2009.05.008
- S. Oh, K.S. Brammer, Y.S. Julie Li, D.Y. Teng, A.J. Engler, A.J. Teng, S. Chien, S. Jin, Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. U.S.A. 106(7), 2130–2135 (2009). doi:10.1073/pnas.0813200106
- X.L. Zhu, J. Chen, L. Scheideler, R. Reichl, J. Geis-Gerstorfer, Effects of topography and composition of titanium surface oxides on osteoblast responses. Biomaterials 25(18), 4087–4103 (2004). doi:10.1016/j.biomaterials.2003.11.011
- M. Lai, K. Cai, L. Zhao, X. Chen, Y. Hou, Z. Yang, Surface functionalization of TiO2 nanotubes with bone morphogenetic protein 2 and its synergistic effect on the differentiation of mesenchymal stem cells. Biomacromolecules 12(4), 1097–1105 (2011). doi:10.1021/bm1014365
- L.W. Lv, Y.S. Liu, P. Zhang, X. Zhang, J.Z. Liu, T. Chen, P.L. Su, H.Y. Li, Y.S. Zhou, The nanoscale geometry of TiO2 nanotubes influences the osteogenic differentiation of human adipose-derived stem cells by modulating H3K4 trimethylation. Biomaterials 39, 193–205 (2015). doi:10.1016/j.biomaterials.2014.11.002
- W.W. Liu, P. Su, S. Chen, N. Wang, Y.P. Ma, Y.R. Liu, J.S. Wang, Z.T. Zhang, H.Y. Li, T.J. Webster, Synthesis of TiO2 nanotubes with ZnO nanoparticles to achieve antibacterial properties and stem cell compatibility. Nanoscale 6, 9050–9062 (2014). doi:10.1039/C4NR01531B
- N. Wang, H. Li, W. Lu, J. Li, J. Wang, Z. Zhang, Y. Liu, Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials 32(29), 6900–6911 (2011). doi:10.1016/j.biomaterials.2011.06.023
- R.P. Zhang, H.L. Wu, J.H. Ni, C.L. Zhao, Y.F. Chen, C.J.Y. Zheng, X.N. Zhang, Guided proliferation and bone-forming functionality on highly ordered large diameter TiO2 nanotube arrays. Mater. Sci. Eng. C 53, 272–279 (2015). doi:10.1016/j.msec.2015.04.046
- J.H. Ni, K. Noh, C.J. Frandsen, S.D. Kong, G. He, T.T. Tang, S. Jin, Preparation of near micrometer-sized TiO2 nanotube arrays by high voltage anodization. Mater. Sci. Eng. C 33(1), 259–264 (2013). doi:10.1016/j.msec.2012.08.038
- T. Ishizaki, N. Saito, O. Takai, Correlation of cell adhesive behaviors on superhydrophobic, superhydrophilic, and micropatterned superhydrophobic/superhydrophilic surfaces to their surface chemistry. Langmuir 26(11), 8147–8154 (2010). doi:10.1021/la904447c
- K. Anselme, Osteoblast adhesion on biomaterials. Biomaterials 21(7), 667–681 (2000). doi:10.1016/S0142-9612(99)00242-2
- C. Galli, M.C. Coen, R. Hauert, V.L. Katanaev, M.P. Wymann, P. Groning, L. Schlapbach, Protein adsorption on topographically nanostructured titanium. Surf. Sci. 474(1–3), L180–L184 (2001). doi:10.1016/S0039-6028(00)01054-2
References
M. Ventre, F. Cause, P.A. Netti, Determinants of cell-material crosstalk at the interface: towards engineering of cell instructive materials. J. R. Soc. Interface 9, 2017–2032 (2012). doi:10.1098/rsif.2012.0308
J.T. Parsons, A.R. Horwitz, M.A. Schwartz, Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11, 633–643 (2010). doi:10.1038/nrm2957
T.W. Qin, Z.M. Yang, Z.Z. Wu, H.Q. Xie, J. Qin, S.X. Cai, Adhesion strength of human tenocytes to extracellular matrix component-modified poly(dl-lactide-co-glycolide) substrates. Biomaterials 26(33), 6635–6642 (2005). doi:10.1016/j.biomaterials.2005.04.023
Z. Gao, S. Wang, H. Zhu, C.N. Su, G.L. Xu, X.J. Lian, Using selected uniform cells in round shape with a micropipette to measure cell adhesion strength on silk fibroin-based materials. Mater. Sci. Eng. C 28(8), 1227–1235 (2008). doi:10.1016/j.msec.2007.11.003
K. Anselme, M. Bigerelle, Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomater. 1(2), 211–222 (2005). doi:10.1016/j.actbio.2004.11.009
D. Regonini, C.R. Bowen, A. Jaroenworaluck, R. Stevens, A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 74(12), 377–406 (2013). doi:10.1016/j.mser.2013.10.001
G.K. Mor, O.K. Varghese, M. Paulose, K. Shankar, C.A. Grimes, A review on highly ordered, vertically oriented TiO2 nanotube arrays: fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 90(14), 2011–2075 (2006). doi:10.1016/j.solmat.2006.04.007
E. Gultepe, D. Nagesha, S. Sridhar, M. Amiji, Nanoporous inorganic membranes or coatings for sustained drug delivery in implantable devices. Adv. Drug Del. Rev. 62, 305–315 (2010). doi:10.1016/j.addr.2009.11.003
A. Kodama, S. Bauer, A. Komatsu, H. Asoh, S. Ono, P. Schmuki, Bioactivation of titanium surfaces using coatings of TiO2 nanotubes rapidly pre-loaded with synthetic hydroxyapatite. Acta Biomater. 5(6), 2322–2330 (2009). doi:10.1016/j.actbio.2009.02.032
S.P. Albu, A. Ghicov, S. Aldabergenova, P. Drechsel, D.J. LeClere, G.E. Thompson, J.M. Macak, P. Schmuki, Formation of double-walled TiO2 nanotubes and robust anatase membranes. Adv. Mater. 20(21), 4135–4139 (2008). doi:10.1002/adma.200801189
H. Yin, H. Liu, W.Z. Shen, The large diameter and fast growth of self-organized TiO2 nanotube arrays achieved via electrochemical anodization. Nanotechnology 21, 035601 (2010). doi:10.1088/0957-4484/21/3/035601
S. Oh, C. Daraio, L.H. Chen, T.R. Pisantic, R.R. Finones, S. Jin, Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J. Biomed. Mater. Res. A 78(1), 97–103 (2006). doi:10.1002/jbm.a.30722
K. Das, S. Bose, A. Bandyopadhyay, TiO2 nanotubes on Ti: influence of nanoscale morphology on bone cell-materials interaction. J. Biomed. Mater. Res. A 90(1), 225–237 (2009). doi:10.1002/jbm.a.32088
S. Oh, S. Jin, Titanium oxide nanotubes with controlled morphology for enhanced bone growth. Mater. Sci. Eng. C 26(8), 1301–1306 (2006). doi:10.1016/j.msec.2005.08.014
K.S. Brammer, C.J. Frandsen, S. Jin, TiO2 nanotubes for bone regeneration. Trends Biotechnol. 30(6), 315–322 (2012). doi:10.1016/j.tibtech.2012.02.005
J.H. Ni, C.J. Frandsen, K. Noh, G.W. Johnston, G. He, T.T. Tang, S. Jin, Fabrication of thin film TiO2 nanotube arrays on Co-28Cr-6Mo alloy by anodization. Mater. Sci. Eng. C 33, 1460–1466 (2013). doi:10.1016/j.msec.2012.12.068
J. Park, S. Bauer, V.D. Mark, P. Schmuki, Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 7(6), 1686–1691 (2007). doi:10.1021/nl070678d
J. Park, S. Bauer, K.A. Schlegel, F.W. Neukam, K. Von der Mark, P. Schmuki, TiO2 nanotube surfaces: 15 nm—an optimal length scale of surface topography for cell adhesion and differentiation. Small 5(6), 666–671 (2009). doi:10.1002/smll.200801476
K.S. Brammer, S. Oh, C.J. Cobb, L.M. Bjursten, H.V.D. Heyde, S. Jin, Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 5(8), 3215–3223 (2009). doi:10.1016/j.actbio.2009.05.008
S. Oh, K.S. Brammer, Y.S. Julie Li, D.Y. Teng, A.J. Engler, A.J. Teng, S. Chien, S. Jin, Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. U.S.A. 106(7), 2130–2135 (2009). doi:10.1073/pnas.0813200106
X.L. Zhu, J. Chen, L. Scheideler, R. Reichl, J. Geis-Gerstorfer, Effects of topography and composition of titanium surface oxides on osteoblast responses. Biomaterials 25(18), 4087–4103 (2004). doi:10.1016/j.biomaterials.2003.11.011
M. Lai, K. Cai, L. Zhao, X. Chen, Y. Hou, Z. Yang, Surface functionalization of TiO2 nanotubes with bone morphogenetic protein 2 and its synergistic effect on the differentiation of mesenchymal stem cells. Biomacromolecules 12(4), 1097–1105 (2011). doi:10.1021/bm1014365
L.W. Lv, Y.S. Liu, P. Zhang, X. Zhang, J.Z. Liu, T. Chen, P.L. Su, H.Y. Li, Y.S. Zhou, The nanoscale geometry of TiO2 nanotubes influences the osteogenic differentiation of human adipose-derived stem cells by modulating H3K4 trimethylation. Biomaterials 39, 193–205 (2015). doi:10.1016/j.biomaterials.2014.11.002
W.W. Liu, P. Su, S. Chen, N. Wang, Y.P. Ma, Y.R. Liu, J.S. Wang, Z.T. Zhang, H.Y. Li, T.J. Webster, Synthesis of TiO2 nanotubes with ZnO nanoparticles to achieve antibacterial properties and stem cell compatibility. Nanoscale 6, 9050–9062 (2014). doi:10.1039/C4NR01531B
N. Wang, H. Li, W. Lu, J. Li, J. Wang, Z. Zhang, Y. Liu, Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials 32(29), 6900–6911 (2011). doi:10.1016/j.biomaterials.2011.06.023
R.P. Zhang, H.L. Wu, J.H. Ni, C.L. Zhao, Y.F. Chen, C.J.Y. Zheng, X.N. Zhang, Guided proliferation and bone-forming functionality on highly ordered large diameter TiO2 nanotube arrays. Mater. Sci. Eng. C 53, 272–279 (2015). doi:10.1016/j.msec.2015.04.046
J.H. Ni, K. Noh, C.J. Frandsen, S.D. Kong, G. He, T.T. Tang, S. Jin, Preparation of near micrometer-sized TiO2 nanotube arrays by high voltage anodization. Mater. Sci. Eng. C 33(1), 259–264 (2013). doi:10.1016/j.msec.2012.08.038
T. Ishizaki, N. Saito, O. Takai, Correlation of cell adhesive behaviors on superhydrophobic, superhydrophilic, and micropatterned superhydrophobic/superhydrophilic surfaces to their surface chemistry. Langmuir 26(11), 8147–8154 (2010). doi:10.1021/la904447c
K. Anselme, Osteoblast adhesion on biomaterials. Biomaterials 21(7), 667–681 (2000). doi:10.1016/S0142-9612(99)00242-2
C. Galli, M.C. Coen, R. Hauert, V.L. Katanaev, M.P. Wymann, P. Groning, L. Schlapbach, Protein adsorption on topographically nanostructured titanium. Surf. Sci. 474(1–3), L180–L184 (2001). doi:10.1016/S0039-6028(00)01054-2