Self-assembly Polyrotaxanes Nanoparticles as Carriers for Anticancer Drug Methotrexate Delivery
Corresponding Author: Zhongwei Gu
Nano-Micro Letters,
Vol. 6 No. 2 (2014), Article Number: 108-115
Abstract
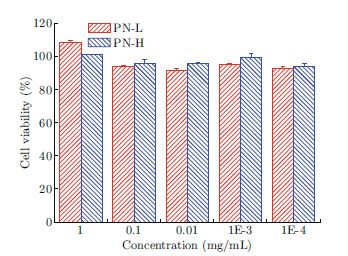
α-Cyclodextrin/poly(ethylene glycol) (α-CD/PEG) polyrotaxane nanoparticles were prepared via a self-assembly method. Anticancer drug methotrexate (MTX) was loaded in the nanoparticles. The interaction between MTX and polyrotaxane was investigated. The formation, morphology, drug release and in vitro anticancer activity of the MTX loaded polyrotaxane nanoparticles were studied. The results show that the MTX could be efficiently absorbed on the nanoparticles, and hydrogen bonds were formed between MTX and α-CDs. The typical channel-type stacking assembly style of polyrotaxane nanoparticles was changed after MTX was loaded. The mean diameter of drug loaded polyrotaxane nanoparticles were around 200 nm and the drug loading content was as high as about 20%. Drug release profiles show that most of the loaded MTX was released within 8 hours and the cumulated release rate was as high as 98%. The blank polyrotaxane nanoparticles were nontoxicity to cells. The in vitro anticancer activity of the MTX loaded polyrotaxane nanoparticles was higher than that of free MTX.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- C. C. Rusa, T. A. Bullions, J. Fox, F. E. Porbeni, X. Wang and A. E. Tonelli, “Inclusion compound formation with a new columnar cyclodextrin host”, Langmuir 18(25), 10016–10023 (2002). http://dx.doi.org/10.1021/la0262452
- I. N. Topchieva, A. E. Tonelli, I. G. Panova, E. V. Matuchina, F. A. Kalashnikov, V. I. Gerasimov, C. C. Rusa, M. Rusa and M. A. Hunt, “Two-phase channel structures based on λ-cyclodextrin-polyethylene glycol inclusion complexes”, Langmuir 20(21), 9036–9043 (2004). http://dx.doi.org/10.1021/la048970d
- A. Harada and M. Kamachi, “Complex formation between poly(ethylene glycol) and α-cyclodextrin”, Macromolecules 23(10), 2821–2823 (1990). http://dx.doi.org/10.1021/ma00212a039
- J. Li, X. Ni, Z. Zhou and K. W. Leong, “Preparation and characterization of polypseudorotaxanes based on block-selected inclusion complexation between poly(propylene oxide)-poly(ethylene oxide)-poly(propylene oxide) triblock copolymers and α-cyclodextrin”, J. Am. Chem. Soc. 125(7), 1788–1795 (2003). http://dx.doi.org/10.1021/ja026623p
- J. J. Li, F. Zhao and J. Li, “Polyrotaxanes for applications in life science and biotechnology”, Appl. Microbiol. Biotechnol. 90(2), 427–443 (2011). http://dx.doi.org/10.1007/s00253-010-3037-x
- C. Moon, Y. M. Kwon, W. K. Lee, Y. J. Park and V. C. Yang, “In vitro assessment of a novel polyrotaxane-based drug delivery system integrated with a cell-penetrating peptide”, J. Controlled Release 124(1–2), 43–50 (2007). http://dx.doi.org/10.1016/j.jconrel.2007.08.029
- C. Moon, Y. M. Kwon, W. K. Lee, Y. J. Park, L. C. Chang and V. C. Yang, “A novel polyrotaxane-based intracellular delivery system for camptothecin: In vitro feasibility evaluation”, J. Biomed. Mater. Res. 84A(1), 238–246 (2008). http://dx.doi.org/10.1002/jbm.a.31452
- N. Yui and T. Ooya, “Molecular mobility of interlocked structures exploiting new functions of advanced biomaterials”, Chem. Eur. J. 12(26), 6730–6737 (2006). http://dx.doi.org/10.1002/chem.200600370
- N. Yui, R. Katoono and A. Yamashita, “Functional cyclodextrinpolyrotaxanes for drug delivery”, Adv. Polym. Sci. 222, 115–173 (2009). http://dx.doi.org/10.1007/12_2008_8
- T. Ooya, H. S. Choi, A. Yamashita, N. Yui, Y. Sugaya, A. Kano, A. Maruyama, H. Akita, R. Ito, K. Kogure and H. Harashima, “Biocleavablepolyrotaxane-plasmid DNA polyplex for enhanced gene delivery”, J. Am. Chem. Soc. 128(12), 3852–3853 (2006). http://dx.doi.org/10.1021/ja055868+
- J. Li, C. Yang, H. Z. Li, X. Wang, S. H. Goh, J. L. Ding, D. Y. Wang and K. W. Leong, “Cationic supramolecules composed of multiple, oligoethylenimine-grafted beta-cyclodextrins threaded on a polymer, chain for efficient gene delivery”, Adv. Mater. 18(22), 2969–2974 (2006). http://dx.doi.org/10.1002/adma.200600812
- L. X. Ren, F. Y. Ke, Y. M. Chen, D. H. Liang and J. Huang, “Supramolecular ABA triblock copolymer with polyrotaxane as B block and its hierarchical selfassembly”, Macromolecules 41(14), 5295–5300 (2008). http://dx.doi.org/10.1021/ma800632m
- J. Huang, L. X. Ren and Y. M. Chen, “pH/temperature-sensitive supramolecular micelles based on cyclodextrinpolyrotaxane”, Polym. Int. 57(5), 714–721 (2008). http://dx.doi.org/10.1002/pi.2396
- C. C. Tsai, S. Leng, K. Jeong, V. R. M. Horn, C. L. Wang, W. B. Zhang, M. J. Graham, J. Huang, R. M. Ho, Y. Chen, B. Lotz and S. Z. D. Cheng, “Supramolecular structure of β-cyclodextrin and poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) inclusion complexes”, Macromolecules 43(22), 9454–9461 (2010). http://dx.doi.org/10.1021/ma101943k
- J. Chang, Y. Li, G. Wang, B. He and Z. W. Gu, “Fabrication of novel coumarin derivative functionalized polypseudorotaxane micelles for drug delivery”, Nanoscale 5, 813–820 (2013) http://dx.doi.org/10.1039/c2nr32927a
- R. Liu, Y. S. Lai, B. He, Y. Li, G. Wang, S. Chang and Z. W. Gu, “Supramolecular nanoparticles generated by the self-assembly of polyrotaxanes for anti-tumor drug delivery”, Int. J. Nanomed. 7, 5249–5258 (2012). http://dx.doi.org/10.2147/ijn.s33649
- A. Harada, J. Li and M. Kamachi, “The molecular necklace: a rotaxane containing many threaded α-cyclodextrins”, Nature 356, 325–327 (1992). http://dx.doi.org/10.1038/356325a0
- A, Harada, J. Li and M. Kamachi, “Preparation and properties of inclusion complexes of poly(ethylene glycol) with α-cyclodextrin”, Macromolecules 26(21), 5698–5703 (1993). http://dx.doi.org/10.1021/ma00073a026
- T. Uyar, M. A. Hunt, H. S. Gracz and A. E. Tonelli, “Crystalline cyclodextrin inclusion compounds formed with aromatic guests:guest-dependent stoichiometries and hydration-sensitive crystal stuctures”, Cryst. Growth Des. 6(5), 1113–1119 (2006). http://dx.doi.org/10.1021/cg050500+
- Y. Lai, Y. Lei, X. Xu, Y. Li, B. He and Z. Gu, “Polymeric micelles with π-π conjugated cinnamic acid as lipophilic moieties for doxorubicin delivery”, J. Mater Chem. B 1, 4289–4296 (2013). http://dx.doi.org/10.1039/c3tb20392a
- S. Pan, S. Luo, S. Li., Y. Lai, Y. Geng, B. He. and Z. Gu, “Ultrasound accelerated gelation of novel L-lysine based hydrogelators”, Chem. Comm. 49, 8045–8047 (2013). http://dx.doi.org/10.1039/c3cc44767g
- B. He and M. B. Chan-Park, “Synthesis and charaterization of functionalizable, biodegradable and photopatternablepoly(ε-caprolactone-co-RS-β-malic acid)”, Macromolecule 38(20), 8227–8234 (2005). http://dx.doi.org/10.1021/ma050545j
- Y. Lei, Y. Lai, Y. Li, S. Li, G. Cheng, D. Li, H. Li, B. He and Z. Gu, “Anticancer drug delivery of PEG based micelles with small lipophilic moieties”, Int. J. Pharm. 453(2), 579–586 (2013). http://dx.doi.org/10.1016/j.ijpharm.2013.06.001
- D. Li, Y. Liang, Y. Lai, G. Wang, B. He and Z. Gu, “Polymeric micelles with small lipophilic moieties for drug delivery”, Colloids Surf. B: Biointerface http://dx.doi.org/10.1016/j.colsurfb.2013.10.032
- B. Xue, Y. Wang, X. Tang, P. Xie, Y. Wang, F. Luo, C. Wu and Z. Qian, “Biodegradable self-assembled mPEG-PCL micelles for hydrophobic oridonin delivery in vitro”, J. Biomed. Nanotechnol. 8(1), 80–89 (2012). http://dx.doi.org/10.1166/jbn.2012.1358
- M. Gou, K. Men, H. Shi, M. Xiang, J. Zhang, J. Song, J. Long, Y. Wan, F. Luo, X. Zhao and Z. Qian, “Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo”, Nanoscale 3, 1558–1567 (2011). http://dx.doi.org/10.1039/c0nr00758g
- X. Deng, X. Xu, Y. Lai, B. He and Z. Gu, “Novel nanoparticles generated by polymeric amphiphiles with π-π conjugated small molecules for anti-tumor drug delivery”, J. Biomed. Nanotechnol. 9(8), 1336–1344 (2013). http://dx.doi.org/10.1166/jbn.2013.1626
References
C. C. Rusa, T. A. Bullions, J. Fox, F. E. Porbeni, X. Wang and A. E. Tonelli, “Inclusion compound formation with a new columnar cyclodextrin host”, Langmuir 18(25), 10016–10023 (2002). http://dx.doi.org/10.1021/la0262452
I. N. Topchieva, A. E. Tonelli, I. G. Panova, E. V. Matuchina, F. A. Kalashnikov, V. I. Gerasimov, C. C. Rusa, M. Rusa and M. A. Hunt, “Two-phase channel structures based on λ-cyclodextrin-polyethylene glycol inclusion complexes”, Langmuir 20(21), 9036–9043 (2004). http://dx.doi.org/10.1021/la048970d
A. Harada and M. Kamachi, “Complex formation between poly(ethylene glycol) and α-cyclodextrin”, Macromolecules 23(10), 2821–2823 (1990). http://dx.doi.org/10.1021/ma00212a039
J. Li, X. Ni, Z. Zhou and K. W. Leong, “Preparation and characterization of polypseudorotaxanes based on block-selected inclusion complexation between poly(propylene oxide)-poly(ethylene oxide)-poly(propylene oxide) triblock copolymers and α-cyclodextrin”, J. Am. Chem. Soc. 125(7), 1788–1795 (2003). http://dx.doi.org/10.1021/ja026623p
J. J. Li, F. Zhao and J. Li, “Polyrotaxanes for applications in life science and biotechnology”, Appl. Microbiol. Biotechnol. 90(2), 427–443 (2011). http://dx.doi.org/10.1007/s00253-010-3037-x
C. Moon, Y. M. Kwon, W. K. Lee, Y. J. Park and V. C. Yang, “In vitro assessment of a novel polyrotaxane-based drug delivery system integrated with a cell-penetrating peptide”, J. Controlled Release 124(1–2), 43–50 (2007). http://dx.doi.org/10.1016/j.jconrel.2007.08.029
C. Moon, Y. M. Kwon, W. K. Lee, Y. J. Park, L. C. Chang and V. C. Yang, “A novel polyrotaxane-based intracellular delivery system for camptothecin: In vitro feasibility evaluation”, J. Biomed. Mater. Res. 84A(1), 238–246 (2008). http://dx.doi.org/10.1002/jbm.a.31452
N. Yui and T. Ooya, “Molecular mobility of interlocked structures exploiting new functions of advanced biomaterials”, Chem. Eur. J. 12(26), 6730–6737 (2006). http://dx.doi.org/10.1002/chem.200600370
N. Yui, R. Katoono and A. Yamashita, “Functional cyclodextrinpolyrotaxanes for drug delivery”, Adv. Polym. Sci. 222, 115–173 (2009). http://dx.doi.org/10.1007/12_2008_8
T. Ooya, H. S. Choi, A. Yamashita, N. Yui, Y. Sugaya, A. Kano, A. Maruyama, H. Akita, R. Ito, K. Kogure and H. Harashima, “Biocleavablepolyrotaxane-plasmid DNA polyplex for enhanced gene delivery”, J. Am. Chem. Soc. 128(12), 3852–3853 (2006). http://dx.doi.org/10.1021/ja055868+
J. Li, C. Yang, H. Z. Li, X. Wang, S. H. Goh, J. L. Ding, D. Y. Wang and K. W. Leong, “Cationic supramolecules composed of multiple, oligoethylenimine-grafted beta-cyclodextrins threaded on a polymer, chain for efficient gene delivery”, Adv. Mater. 18(22), 2969–2974 (2006). http://dx.doi.org/10.1002/adma.200600812
L. X. Ren, F. Y. Ke, Y. M. Chen, D. H. Liang and J. Huang, “Supramolecular ABA triblock copolymer with polyrotaxane as B block and its hierarchical selfassembly”, Macromolecules 41(14), 5295–5300 (2008). http://dx.doi.org/10.1021/ma800632m
J. Huang, L. X. Ren and Y. M. Chen, “pH/temperature-sensitive supramolecular micelles based on cyclodextrinpolyrotaxane”, Polym. Int. 57(5), 714–721 (2008). http://dx.doi.org/10.1002/pi.2396
C. C. Tsai, S. Leng, K. Jeong, V. R. M. Horn, C. L. Wang, W. B. Zhang, M. J. Graham, J. Huang, R. M. Ho, Y. Chen, B. Lotz and S. Z. D. Cheng, “Supramolecular structure of β-cyclodextrin and poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) inclusion complexes”, Macromolecules 43(22), 9454–9461 (2010). http://dx.doi.org/10.1021/ma101943k
J. Chang, Y. Li, G. Wang, B. He and Z. W. Gu, “Fabrication of novel coumarin derivative functionalized polypseudorotaxane micelles for drug delivery”, Nanoscale 5, 813–820 (2013) http://dx.doi.org/10.1039/c2nr32927a
R. Liu, Y. S. Lai, B. He, Y. Li, G. Wang, S. Chang and Z. W. Gu, “Supramolecular nanoparticles generated by the self-assembly of polyrotaxanes for anti-tumor drug delivery”, Int. J. Nanomed. 7, 5249–5258 (2012). http://dx.doi.org/10.2147/ijn.s33649
A. Harada, J. Li and M. Kamachi, “The molecular necklace: a rotaxane containing many threaded α-cyclodextrins”, Nature 356, 325–327 (1992). http://dx.doi.org/10.1038/356325a0
A, Harada, J. Li and M. Kamachi, “Preparation and properties of inclusion complexes of poly(ethylene glycol) with α-cyclodextrin”, Macromolecules 26(21), 5698–5703 (1993). http://dx.doi.org/10.1021/ma00073a026
T. Uyar, M. A. Hunt, H. S. Gracz and A. E. Tonelli, “Crystalline cyclodextrin inclusion compounds formed with aromatic guests:guest-dependent stoichiometries and hydration-sensitive crystal stuctures”, Cryst. Growth Des. 6(5), 1113–1119 (2006). http://dx.doi.org/10.1021/cg050500+
Y. Lai, Y. Lei, X. Xu, Y. Li, B. He and Z. Gu, “Polymeric micelles with π-π conjugated cinnamic acid as lipophilic moieties for doxorubicin delivery”, J. Mater Chem. B 1, 4289–4296 (2013). http://dx.doi.org/10.1039/c3tb20392a
S. Pan, S. Luo, S. Li., Y. Lai, Y. Geng, B. He. and Z. Gu, “Ultrasound accelerated gelation of novel L-lysine based hydrogelators”, Chem. Comm. 49, 8045–8047 (2013). http://dx.doi.org/10.1039/c3cc44767g
B. He and M. B. Chan-Park, “Synthesis and charaterization of functionalizable, biodegradable and photopatternablepoly(ε-caprolactone-co-RS-β-malic acid)”, Macromolecule 38(20), 8227–8234 (2005). http://dx.doi.org/10.1021/ma050545j
Y. Lei, Y. Lai, Y. Li, S. Li, G. Cheng, D. Li, H. Li, B. He and Z. Gu, “Anticancer drug delivery of PEG based micelles with small lipophilic moieties”, Int. J. Pharm. 453(2), 579–586 (2013). http://dx.doi.org/10.1016/j.ijpharm.2013.06.001
D. Li, Y. Liang, Y. Lai, G. Wang, B. He and Z. Gu, “Polymeric micelles with small lipophilic moieties for drug delivery”, Colloids Surf. B: Biointerface http://dx.doi.org/10.1016/j.colsurfb.2013.10.032
B. Xue, Y. Wang, X. Tang, P. Xie, Y. Wang, F. Luo, C. Wu and Z. Qian, “Biodegradable self-assembled mPEG-PCL micelles for hydrophobic oridonin delivery in vitro”, J. Biomed. Nanotechnol. 8(1), 80–89 (2012). http://dx.doi.org/10.1166/jbn.2012.1358
M. Gou, K. Men, H. Shi, M. Xiang, J. Zhang, J. Song, J. Long, Y. Wan, F. Luo, X. Zhao and Z. Qian, “Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo”, Nanoscale 3, 1558–1567 (2011). http://dx.doi.org/10.1039/c0nr00758g
X. Deng, X. Xu, Y. Lai, B. He and Z. Gu, “Novel nanoparticles generated by polymeric amphiphiles with π-π conjugated small molecules for anti-tumor drug delivery”, J. Biomed. Nanotechnol. 9(8), 1336–1344 (2013). http://dx.doi.org/10.1166/jbn.2013.1626