Polymer Nanoparticles Prepared by Supercritical Carbon Dioxide for in Vivo Anti-cancer Drug Delivery
Corresponding Author: Xiufu Hua
Nano-Micro Letters,
Vol. 6 No. 1 (2014), Article Number: 20-23
Abstract
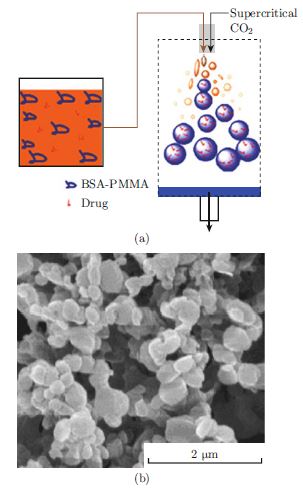
A new approach for producing polymer nanoparticles made of bovine serum albumin-poly(methyl methacrylate) conjugate by precipitating in supercritical CO2 is reported. The nanoparticles were loaded with the anti-tumor drug camptothecin. With albumin serving as a nutrient to cells, the drug-encapsulated nanoparticle shows an enhanced ability to kill cancer cells compared to that of the free drug in solution both in vitro and in vivo.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- L. Zhang, F. Gu, J. Chan, A. Wang, R. S. Langer and O. C. Farokhzad, “Nanoparticles in medicine: therapeutic applications and developments”, Clin. Pharmacol. Ther. 83(5), 761–769 (2008). http://dx.doi.org/10.1038/sj.clpt.6100400
- R. Wang, Y. Zhang, D. Lu, J. Ge, Z. Liu and R. N. Zare, “Functional protein-organic/inorganic hybrid nanomaterials”, WIERs: Nanomed. Nanobiotech. 5(4), 320–328 (2013). http://dx.doi.org/10.1002/wnan.1210
- J. Ge, E. Neofytou, J. Lei, R. E. Beygui and R. N. Zare, “Protein-polymer hybrid nanoparticles for drug delivery”, Small 8(23), 3573–3578 (2012). http://dx.doi.org/10.1002/smll.201200889
- J. Ge, E. Neofytou, T. J. Cahill III, R. E. Beygui and R. N. Zare, “Drug release from electric-field-responsive nanoparticles”, ACS Nano 6(1), 227–233 (2012). http://dx.doi.org/10.1021/nn203430m
- J. Ge, J. Lei and R. N. Zare, “Bovine serum albumin-poly(methyl methacrylate) nanoparticles: an example of frustrated phase separation”, Nano Lett. 11(6), 2551–2554 (2011). http://dx.doi.org/10.1021/nl201303q
- J. Ge, G. B. Jacobson, T. Lobovkina, K. Holmberg and R. N. Zare, “Sustained release of nucleic acids from polymeric nanoparticles using microemulsion precipitation in supercritical carbon dioxide”, Chem. Comm. 46(47), 9034–9036 (2010). http://dx.doi.org/10.1039/c0cc04258g
- B. C. Giovanella, J. S. Stehlin, M. E. Wall, M. C. Wani, A. W. Nicholas, L. F. Liu, R. Silber and M. Potmesil, “DNA topoisomerase I—targeted chemotherapy of human colon cancer in xenografts”, Science 246(4933), 1046–1048 (1989). http://dx.doi.org/10.1126/science.2555920
- B. Ertl, P. Platzer, M. Wirth and F. Gabor, “Poly(D,L-lactic-co-glycolic acid) microspheres for sustained delivery and stabilization of camptothecin”, J. Controlled Release 61(3), 305–317 (1999). http://dx.doi.org/10.1016/S0168-3659(99)00122-4
- C. L. Dora, M. Alvarez-Silva, A. G. Trentin, T. J. de Faria, D. Fernandes, R. da Costa, M. Stimamiglio and E. Lemos-Senna, “Evaluation of antimetastatic activity and systemic toxicity of camptothecinloaded microspheres in mice injected with B16-F10 melanoma cells”, J. Pharm. Pharm. Sci. 9(1), 22–31 (2006). http://www.ualberta.ca/$¡m$csps/JPPS9(1)/Senna.E/B16-F10.htm
- M. Ferrari, M. C. Fornasiero and A. M. Isetta, “MTT colorimetric assay for testing macrophage cytotoxic activity in vitro”, J. Immunol. Methods 131(2), 165–172 (1990). http://dx.doi.org/10.1016/0022-1759(90)90187-Z
- J. Cheng, K. T. Khin and M. E. Davis, “Antitumor activity of beta-cyclodextrin polymer-camptothecin conjugates”, Mol. Pharm. 1(3), 183–193 (2004). http://dx.doi.org/10.1021/mp049966y
- A. Bettencourt and A. J. Almeida, “Poly(methyl methacrylate) particulate carriers in drug delivery”, J. Microencapsulation 29(4), 353–367 (2012). http://dx.doi.org/10.3109/02652048.2011.651500
- C. Passirani, G. Barratt, J. P. Devissaguet and D. Labarre, “Long-circulating nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate)”, Pharm. Res. 15(7), 1046–1050 (1998). http://dx.doi.org/10.1023/A:1011930127562
References
L. Zhang, F. Gu, J. Chan, A. Wang, R. S. Langer and O. C. Farokhzad, “Nanoparticles in medicine: therapeutic applications and developments”, Clin. Pharmacol. Ther. 83(5), 761–769 (2008). http://dx.doi.org/10.1038/sj.clpt.6100400
R. Wang, Y. Zhang, D. Lu, J. Ge, Z. Liu and R. N. Zare, “Functional protein-organic/inorganic hybrid nanomaterials”, WIERs: Nanomed. Nanobiotech. 5(4), 320–328 (2013). http://dx.doi.org/10.1002/wnan.1210
J. Ge, E. Neofytou, J. Lei, R. E. Beygui and R. N. Zare, “Protein-polymer hybrid nanoparticles for drug delivery”, Small 8(23), 3573–3578 (2012). http://dx.doi.org/10.1002/smll.201200889
J. Ge, E. Neofytou, T. J. Cahill III, R. E. Beygui and R. N. Zare, “Drug release from electric-field-responsive nanoparticles”, ACS Nano 6(1), 227–233 (2012). http://dx.doi.org/10.1021/nn203430m
J. Ge, J. Lei and R. N. Zare, “Bovine serum albumin-poly(methyl methacrylate) nanoparticles: an example of frustrated phase separation”, Nano Lett. 11(6), 2551–2554 (2011). http://dx.doi.org/10.1021/nl201303q
J. Ge, G. B. Jacobson, T. Lobovkina, K. Holmberg and R. N. Zare, “Sustained release of nucleic acids from polymeric nanoparticles using microemulsion precipitation in supercritical carbon dioxide”, Chem. Comm. 46(47), 9034–9036 (2010). http://dx.doi.org/10.1039/c0cc04258g
B. C. Giovanella, J. S. Stehlin, M. E. Wall, M. C. Wani, A. W. Nicholas, L. F. Liu, R. Silber and M. Potmesil, “DNA topoisomerase I—targeted chemotherapy of human colon cancer in xenografts”, Science 246(4933), 1046–1048 (1989). http://dx.doi.org/10.1126/science.2555920
B. Ertl, P. Platzer, M. Wirth and F. Gabor, “Poly(D,L-lactic-co-glycolic acid) microspheres for sustained delivery and stabilization of camptothecin”, J. Controlled Release 61(3), 305–317 (1999). http://dx.doi.org/10.1016/S0168-3659(99)00122-4
C. L. Dora, M. Alvarez-Silva, A. G. Trentin, T. J. de Faria, D. Fernandes, R. da Costa, M. Stimamiglio and E. Lemos-Senna, “Evaluation of antimetastatic activity and systemic toxicity of camptothecinloaded microspheres in mice injected with B16-F10 melanoma cells”, J. Pharm. Pharm. Sci. 9(1), 22–31 (2006). http://www.ualberta.ca/$¡m$csps/JPPS9(1)/Senna.E/B16-F10.htm
M. Ferrari, M. C. Fornasiero and A. M. Isetta, “MTT colorimetric assay for testing macrophage cytotoxic activity in vitro”, J. Immunol. Methods 131(2), 165–172 (1990). http://dx.doi.org/10.1016/0022-1759(90)90187-Z
J. Cheng, K. T. Khin and M. E. Davis, “Antitumor activity of beta-cyclodextrin polymer-camptothecin conjugates”, Mol. Pharm. 1(3), 183–193 (2004). http://dx.doi.org/10.1021/mp049966y
A. Bettencourt and A. J. Almeida, “Poly(methyl methacrylate) particulate carriers in drug delivery”, J. Microencapsulation 29(4), 353–367 (2012). http://dx.doi.org/10.3109/02652048.2011.651500
C. Passirani, G. Barratt, J. P. Devissaguet and D. Labarre, “Long-circulating nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate)”, Pharm. Res. 15(7), 1046–1050 (1998). http://dx.doi.org/10.1023/A:1011930127562