Novel Nano-composites SDC–LiNaSO4 as Functional Layer for ITSOFC
Corresponding Author: Yi-Mei Yin
Nano-Micro Letters,
Vol. 7 No. 3 (2015), Article Number: 268-275
Abstract
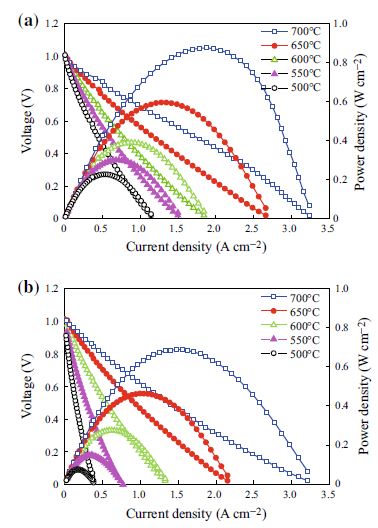
As an ionic conductive functional layer of intermediate temperature solid oxide fuel cells (ITSOFC), samarium-doped ceria (SDC)–LiNaSO4 nano-composites were synthesized by a sol–gel method and their properties were investigated. It was found that the content of LiNaSO4 strongly affected the crystal phase, defect concentration, and conductivity of the composites. When the content of LiNaSO4 was 20 wt%, the highest conductivity of the composite was found to be, respectively, 0.22, 0.26, and 0.35 S cm−1 at temperatures of 550, 600, and 700 °C, which are much higher than those of SDC. The peak power density of the single cell using this composite as an interlayer was improved to, respectively, 0.23, 0.39, and 0.88 W cm−2 at 500, 600, and 700 °C comparing with that of the SDC-based cell. Further, the SDC–LiNaSO4(20 wt%)-based cell also displayed better thermal stability according to the performance measurements at 560 °C for 50 h. These results reveal that SDC–LiNaSO4 composite may be a potential good candidate as interlayer for ITSOFC due to its high ionic conductivity and thermal stability.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- N.Q. Minh, Ceramic fuel cells. J. Am. Ceram. Soc. 76(3), 563–588 (1993). doi:10.1111/j.1151-2916.1993.tb03645.x
- S. Singhal, Solid oxide fuel cells for power generation. WIREs Energy Environ. 3(2), 179–194 (2014). doi:10.1002/wene.96
- J.H. Song, M.G. Jung, H.W. Park, H.T. Lim, The effect of fabrication conditions for GDC buffer layer on electrochemical performance of solid oxide fuel cells. Nano-Micro Lett. 5(3), 151–158 (2013). doi:10.1007/BF03353744
- L.D. Fan, C.Y. Wang, M.M. Chen, B. Zhu, Recent development of ceria-based (nano) composite materials for low temperature ceramic fuel cells and electrolyte-free fuel cells. J. Power Sources 234, 154–174 (2013). doi:10.1016/j.jpowsour.2013.01.138
- D.S. Khaerudini, G.Q. Guan, P. Zhang, X.G. Hao, A. Abudula, Prospects of oxide ionic conductivity bismuth vanadate-based solid electrolytes. Rev. Chem. Eng. 30(6), 539–551 (2014). doi:10.1515/revce-2014-0020
- J.W. Yin, Y.M. Yin, J. Lu, C.M. Zhang, N.Q. Minh, W.M. Zhang, Z.F. Ma, Nd0.5Sr0.5Fe0.8Cu0.2O3-delta-xSm(0.2)Ce(0.8)O(1.9) cobalt-free composite cathodes for intermediate temperature solid oxide fuel cells. Int. J. Hydrogen Energy 39(31), 17852–17856 (2014). doi:10.1016/j.ijhydene.2014.08.131
- J.H. Song, M.Y. Park, H.W. Park, H.T. Lim, Single-step preparation of nano-homogeneous NiO/YSZ composite anode for solid oxide fuel cells. Nano-Micro Lett. 5(2), 111–116 (2013). doi:10.1007/BF03353737
- J.W. Yin, Y.M. Yin, J. Lu, C. Zhang, N.Q. Minh, Z.F. Ma, Structure and properties of novel cobalt-free oxides NdxSr1−xFe0.8Cu0.2O3−δ (0.3 ≤ x ≤ 0.7) as cathodes of intermediate temperature solid oxide fuel cells. J. Phys. Chem. C 118(25), 13357–13368 (2014). doi:10.1021/jp500371w
- H. Chen, K. Cheng, F. Ye, W.J. Weng, Preparation and characterization of graded SSC-SDC MIEC cathode for low-temperature solid oxide fuel cells. Ceram. Int. 37(4), 1209–1214 (2011). doi:10.1016/j.ceramint.2010.11.047
- C.R. Xia, M.L. Liu, Novel cathodes for low-temperature solid oxide fuel cells. Adv. Mater. 14(7), 521–523 (2002). doi:10.1002/1521-4095(20020404)14:7<521:AID-ADMA521>3.0.CO;2-C
- W.C. Wu, J.T. Huang, A. Chiba, Synthesis and properties of samaria-doped ceria electrolyte for IT-SOFCs by EDTA-citrate complexing method. J. Power Sources 195(18), 5868–5874 (2010). doi:10.1016/j.jpowsour.2009.12.098
- M. Chen, B.H. Kim, Q. Xu, B.K. Ahn, W.J. Kang, D.P. Huang, Synthesis and electrical properties of Ce0.8Sm0.2O1.9 ceramics for IT-SOFC electrolytes by urea-combustion technique. Ceram. Int. 35(4), 1335–1343 (2009). doi:10.1016/j.ceramint.2008.06.014
- Z.G. Lu, X.D. Zhou, D. Fisher, J. Templeton, J. Stevenson, N.J. Wu, A. Ignatiev, Enhanced performance of an anode-supported YSZ thin electrolyte fuel cell with a laser-deposited Sm0.2Ce0.8O1.9 interlayer. Electrochem. Commun. 12(2), 179–182 (2010). doi:10.1016/j.elecom.2009.11.015
- J. Maier, Nanoionics: ion transport and electrochemical storage in confined systems. Nat. Mater. 4(11), 805–815 (2005). doi:10.1038/nmat1513
- B. Zhu, S. Li, B.E. Mellander, Theoretical approach on ceria-based two-phase electrolytes for low temperature (300–600 °C) solid oxide fuel cells. Electrochem. Commun. 10(2), 302–305 (2008). doi:10.1016/j.elecom.2007.11.037
- Z. Gao, J.B. Huang, Z.Q. Mao, C. Wang, Z.X. Liu, Preparation and characterization of nanocrystalline Ce0.8Sm0.2O1.9 for low temperature solid oxide fuel cells based on composite electrolyte. Int. J. Hydrogen Energy 35(2), 731–737 (2010). doi:10.1016/j.ijhydene.2009.10.090
- C. Xia, Y. Li, Y. Tian, Q.H. Liu, Z.M. Wang, L.J. Jia, Y.C. Zhao, Y.D. Li, Intermediate temperature fuel cell with a doped ceria-carbonate composite electrolyte. J. Power Sources 195(10), 3149–3154 (2010). doi:10.1016/j.jpowsour.2009.11.104
- R. Tarneberg, A. Lunden, Ion diffusion in the high-temperature phases Li2SO4, LiNaSO4, LiAgSO4 and Li4Zn(SO4)3. Solid State Ionics 90, 209–220 (1996). doi:10.1016/S0167-2738(96)00399-2
- J. Lu, Y.M. Yin, Z.F. Ma, Preparation and characterization of new cobalt-free cathode Pr0.5Sr0.5Fe0.8Cu0.2O3-delta for IT-SOFC. Int. J. Hydrogen Energy 38(25), 10527–10533 (2013). doi:10.1016/j.ijhydene.2013.05.164
- S.W. Tai, B. Zhu, D.K. Peng, G.Y. Meng, Investigation on LiNaSO4-Al2O3 ceramics as electrolytes for H2/O2 fuel cells. Mater. Res. Bull. 34(10–11), 1651–1659 (1999). doi:10.1016/S0025-5408(99)00161-0
- K.N. Ganesha, G. Govindaraj, Synthesis, characterization and ion conductivity study of nanocrystalline LiNaSO4. AIP Conf. Proc. 1512, 386–387 (2013). doi:10.1063/1.4791073
- L. Larlsson, R.L. McGreevy, Mechanisms of ionic conduction in Li2SO4 and LiNaSO4 Paddle wheel or percolation? Solid State Ionics 76, 301–308 (1995). doi:10.1016/0167-2738(94)00278-Z
- J. Patakangas, Y. Ma, Y.F. Jing, P. Lund, Review and analysis of characterization methods and ionic conductivities for low-temperature solid oxide fuel cells (LT-SOFC). J. Power Sources 263, 315–331 (2014). doi:10.1016/j.jpowsour.2014.04.008
- Z.L. Zhan, T.L. Wen, H.Y. Tu, Z.Y. Lu, AC impedance investigation of samarium-doped ceria. J. Electrochem. Soc. 148(5), A427–A432 (2001). doi:10.1149/1.1359198
- M. Benamira, A. Ringuede, L. Hildebrandt, C. Lagergren, R.N. Vannier, M. Cassir, Gadolinia-doped ceria mixed with alkali carbonates for SOFC applications: II—an electrochemical insight. Int. J. Hydrogen Energy 37(24), 19371–19379 (2012). doi:10.1016/j.ijhydene.2011.10.062
- J.B. Goodenough, Ceramic technology—oxide-ion conductors by design. Nature 404(6780), 821–823 (2000). doi:10.1038/35009177
- Q.H. Liu, B. Zhu, Theoretical description of superionic conductivities in samaria doped ceria based nanocomposites. Appl. Phys. Lett. 97(18), 183115 (2010). doi:10.1063/1.3513375
- B. Zhu, Functional ceria-salt-composite materials for advanced ITSOFC applications. J. Power Sources 114(1), 1–9 (2003). doi:10.1016/S0378-7753(02)00592-X
- Y.D. Li, Z.B. Rui, C. Xia, M. Anderson, Y.S. Lin, Performance of ionic-conducting ceramic/carbonate composite material as solid oxide fuel cell electrolyte and CO2 permeation membrane. Catal. Today 148(3–4), 303–309 (2009). doi:10.1016/j.cattod.2009.08.009
- Y.F. Ling, J. Patakangas, P.D. Lund, B. Zhu, An improved synthesis method of ceria-carbonate based composite electrolytes for low-temperature SOFC fuel cells. Int. J. Hydrogen Energy 38(36), 16532–16538 (2013). doi:10.1016/j.ijhydene.2013.05.136
- B. Zhu, B.E. Mellander, Proton conduction and diffusion in Li2SO4. Solid State Ionics 97(1–4), 535–540 (1997). doi:10.1016/S0167-2738(97)00057-X
- A. Lunden, B. Mellander, B. Zhu, Mobility of protons and oxygen ions in lithium sulfate and other oxyacid salts. Acta Chem. Scand. 45(9), 981–982 (1991). doi:10.3891/acta.chem.scand.45-0981
- B. Heed, Proton conductivity in fuel cells with solid sulfate electrolytes. Solid State Ionics 46(1–2), 121–125 (1991). doi:10.1016/0167-2738(91)90139-3
- S.Y. Oh, T. Yoshida, G. Kawamura, H. Muto, A. Matsuda, Solid-state mechanochemical synthesis of CsHSO4 and 1,2,4-triazole inorganic-organic composite electrolytes for dry fuel cells. Electrochim. Acta 56(5), 2364–2371 (2011). doi:10.1016/j.electacta.2010.11.035
References
N.Q. Minh, Ceramic fuel cells. J. Am. Ceram. Soc. 76(3), 563–588 (1993). doi:10.1111/j.1151-2916.1993.tb03645.x
S. Singhal, Solid oxide fuel cells for power generation. WIREs Energy Environ. 3(2), 179–194 (2014). doi:10.1002/wene.96
J.H. Song, M.G. Jung, H.W. Park, H.T. Lim, The effect of fabrication conditions for GDC buffer layer on electrochemical performance of solid oxide fuel cells. Nano-Micro Lett. 5(3), 151–158 (2013). doi:10.1007/BF03353744
L.D. Fan, C.Y. Wang, M.M. Chen, B. Zhu, Recent development of ceria-based (nano) composite materials for low temperature ceramic fuel cells and electrolyte-free fuel cells. J. Power Sources 234, 154–174 (2013). doi:10.1016/j.jpowsour.2013.01.138
D.S. Khaerudini, G.Q. Guan, P. Zhang, X.G. Hao, A. Abudula, Prospects of oxide ionic conductivity bismuth vanadate-based solid electrolytes. Rev. Chem. Eng. 30(6), 539–551 (2014). doi:10.1515/revce-2014-0020
J.W. Yin, Y.M. Yin, J. Lu, C.M. Zhang, N.Q. Minh, W.M. Zhang, Z.F. Ma, Nd0.5Sr0.5Fe0.8Cu0.2O3-delta-xSm(0.2)Ce(0.8)O(1.9) cobalt-free composite cathodes for intermediate temperature solid oxide fuel cells. Int. J. Hydrogen Energy 39(31), 17852–17856 (2014). doi:10.1016/j.ijhydene.2014.08.131
J.H. Song, M.Y. Park, H.W. Park, H.T. Lim, Single-step preparation of nano-homogeneous NiO/YSZ composite anode for solid oxide fuel cells. Nano-Micro Lett. 5(2), 111–116 (2013). doi:10.1007/BF03353737
J.W. Yin, Y.M. Yin, J. Lu, C. Zhang, N.Q. Minh, Z.F. Ma, Structure and properties of novel cobalt-free oxides NdxSr1−xFe0.8Cu0.2O3−δ (0.3 ≤ x ≤ 0.7) as cathodes of intermediate temperature solid oxide fuel cells. J. Phys. Chem. C 118(25), 13357–13368 (2014). doi:10.1021/jp500371w
H. Chen, K. Cheng, F. Ye, W.J. Weng, Preparation and characterization of graded SSC-SDC MIEC cathode for low-temperature solid oxide fuel cells. Ceram. Int. 37(4), 1209–1214 (2011). doi:10.1016/j.ceramint.2010.11.047
C.R. Xia, M.L. Liu, Novel cathodes for low-temperature solid oxide fuel cells. Adv. Mater. 14(7), 521–523 (2002). doi:10.1002/1521-4095(20020404)14:7<521:AID-ADMA521>3.0.CO;2-C
W.C. Wu, J.T. Huang, A. Chiba, Synthesis and properties of samaria-doped ceria electrolyte for IT-SOFCs by EDTA-citrate complexing method. J. Power Sources 195(18), 5868–5874 (2010). doi:10.1016/j.jpowsour.2009.12.098
M. Chen, B.H. Kim, Q. Xu, B.K. Ahn, W.J. Kang, D.P. Huang, Synthesis and electrical properties of Ce0.8Sm0.2O1.9 ceramics for IT-SOFC electrolytes by urea-combustion technique. Ceram. Int. 35(4), 1335–1343 (2009). doi:10.1016/j.ceramint.2008.06.014
Z.G. Lu, X.D. Zhou, D. Fisher, J. Templeton, J. Stevenson, N.J. Wu, A. Ignatiev, Enhanced performance of an anode-supported YSZ thin electrolyte fuel cell with a laser-deposited Sm0.2Ce0.8O1.9 interlayer. Electrochem. Commun. 12(2), 179–182 (2010). doi:10.1016/j.elecom.2009.11.015
J. Maier, Nanoionics: ion transport and electrochemical storage in confined systems. Nat. Mater. 4(11), 805–815 (2005). doi:10.1038/nmat1513
B. Zhu, S. Li, B.E. Mellander, Theoretical approach on ceria-based two-phase electrolytes for low temperature (300–600 °C) solid oxide fuel cells. Electrochem. Commun. 10(2), 302–305 (2008). doi:10.1016/j.elecom.2007.11.037
Z. Gao, J.B. Huang, Z.Q. Mao, C. Wang, Z.X. Liu, Preparation and characterization of nanocrystalline Ce0.8Sm0.2O1.9 for low temperature solid oxide fuel cells based on composite electrolyte. Int. J. Hydrogen Energy 35(2), 731–737 (2010). doi:10.1016/j.ijhydene.2009.10.090
C. Xia, Y. Li, Y. Tian, Q.H. Liu, Z.M. Wang, L.J. Jia, Y.C. Zhao, Y.D. Li, Intermediate temperature fuel cell with a doped ceria-carbonate composite electrolyte. J. Power Sources 195(10), 3149–3154 (2010). doi:10.1016/j.jpowsour.2009.11.104
R. Tarneberg, A. Lunden, Ion diffusion in the high-temperature phases Li2SO4, LiNaSO4, LiAgSO4 and Li4Zn(SO4)3. Solid State Ionics 90, 209–220 (1996). doi:10.1016/S0167-2738(96)00399-2
J. Lu, Y.M. Yin, Z.F. Ma, Preparation and characterization of new cobalt-free cathode Pr0.5Sr0.5Fe0.8Cu0.2O3-delta for IT-SOFC. Int. J. Hydrogen Energy 38(25), 10527–10533 (2013). doi:10.1016/j.ijhydene.2013.05.164
S.W. Tai, B. Zhu, D.K. Peng, G.Y. Meng, Investigation on LiNaSO4-Al2O3 ceramics as electrolytes for H2/O2 fuel cells. Mater. Res. Bull. 34(10–11), 1651–1659 (1999). doi:10.1016/S0025-5408(99)00161-0
K.N. Ganesha, G. Govindaraj, Synthesis, characterization and ion conductivity study of nanocrystalline LiNaSO4. AIP Conf. Proc. 1512, 386–387 (2013). doi:10.1063/1.4791073
L. Larlsson, R.L. McGreevy, Mechanisms of ionic conduction in Li2SO4 and LiNaSO4 Paddle wheel or percolation? Solid State Ionics 76, 301–308 (1995). doi:10.1016/0167-2738(94)00278-Z
J. Patakangas, Y. Ma, Y.F. Jing, P. Lund, Review and analysis of characterization methods and ionic conductivities for low-temperature solid oxide fuel cells (LT-SOFC). J. Power Sources 263, 315–331 (2014). doi:10.1016/j.jpowsour.2014.04.008
Z.L. Zhan, T.L. Wen, H.Y. Tu, Z.Y. Lu, AC impedance investigation of samarium-doped ceria. J. Electrochem. Soc. 148(5), A427–A432 (2001). doi:10.1149/1.1359198
M. Benamira, A. Ringuede, L. Hildebrandt, C. Lagergren, R.N. Vannier, M. Cassir, Gadolinia-doped ceria mixed with alkali carbonates for SOFC applications: II—an electrochemical insight. Int. J. Hydrogen Energy 37(24), 19371–19379 (2012). doi:10.1016/j.ijhydene.2011.10.062
J.B. Goodenough, Ceramic technology—oxide-ion conductors by design. Nature 404(6780), 821–823 (2000). doi:10.1038/35009177
Q.H. Liu, B. Zhu, Theoretical description of superionic conductivities in samaria doped ceria based nanocomposites. Appl. Phys. Lett. 97(18), 183115 (2010). doi:10.1063/1.3513375
B. Zhu, Functional ceria-salt-composite materials for advanced ITSOFC applications. J. Power Sources 114(1), 1–9 (2003). doi:10.1016/S0378-7753(02)00592-X
Y.D. Li, Z.B. Rui, C. Xia, M. Anderson, Y.S. Lin, Performance of ionic-conducting ceramic/carbonate composite material as solid oxide fuel cell electrolyte and CO2 permeation membrane. Catal. Today 148(3–4), 303–309 (2009). doi:10.1016/j.cattod.2009.08.009
Y.F. Ling, J. Patakangas, P.D. Lund, B. Zhu, An improved synthesis method of ceria-carbonate based composite electrolytes for low-temperature SOFC fuel cells. Int. J. Hydrogen Energy 38(36), 16532–16538 (2013). doi:10.1016/j.ijhydene.2013.05.136
B. Zhu, B.E. Mellander, Proton conduction and diffusion in Li2SO4. Solid State Ionics 97(1–4), 535–540 (1997). doi:10.1016/S0167-2738(97)00057-X
A. Lunden, B. Mellander, B. Zhu, Mobility of protons and oxygen ions in lithium sulfate and other oxyacid salts. Acta Chem. Scand. 45(9), 981–982 (1991). doi:10.3891/acta.chem.scand.45-0981
B. Heed, Proton conductivity in fuel cells with solid sulfate electrolytes. Solid State Ionics 46(1–2), 121–125 (1991). doi:10.1016/0167-2738(91)90139-3
S.Y. Oh, T. Yoshida, G. Kawamura, H. Muto, A. Matsuda, Solid-state mechanochemical synthesis of CsHSO4 and 1,2,4-triazole inorganic-organic composite electrolytes for dry fuel cells. Electrochim. Acta 56(5), 2364–2371 (2011). doi:10.1016/j.electacta.2010.11.035