Elucidating the Uptake and Distribution of Nanoparticles in Solid Tumors via a Multilayered Cell Culture Model
Corresponding Author: Devika Chithrani
Nano-Micro Letters,
Vol. 7 No. 2 (2015), Article Number: 127-137
Abstract
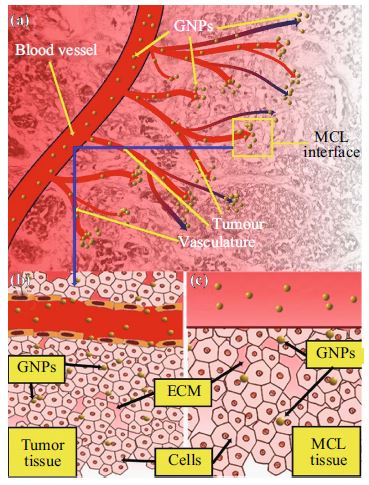
Multicellular layers (MCLs) have previously been used to determine the pharmacokinetics of a variety of different cancer drugs including paclitaxel, doxorubicin, methotrexate, and 5-fluorouracil across a number of cell lines. It is not known how nanoparticles (NPs) navigate through the tumor microenvironment once they leave the tumor blood vessel. In this study, we used the MCL model to study the uptake and penetration dynamics of NPs. Gold nanoparticles (GNPs) were used as a model system to map the NP distribution within tissue-like structures. Our results show that NP uptake and transport are dependent on the tumor cell type. MDA-MB-231 tissue showed deeper penetration of GNPs as compared to MCF-7 one. Intracellular and extracellular distributions of NPs were mapped using CytoViva imaging. The ability of MCLs to mimic tumor tissue characteristics makes them a useful tool in assessing the efficacy of particle distribution in solid tumors.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- J. Lee, L.D.K. Chatterjee, M.H. Lee, I. Krishnan, Gold nanoparticles in breast cancer treatment: promise and potential pitfalls. Cancer Lett. 347(1), 46–53 (2014). doi:10.1016/j.canlet.2014.02.006
- B.D. Chithrani, Optimization of bio-nano interface using gold nanostructures as a model nanoparticle system. Insciences J. 1, 136–156 (2011)
- J.-L. Li, W. Wang, X.-Y. Liu, Z.-P. Zhang, H.-C. Guo, W.-M. Liu, S.-H. Tang, In vitro cancer cell imaging and therapy using transferrin-conjugated gold nanoparticles. Cancer Lett. 274(2), 319–326 (2008). doi:10.1016/j.canlet.2008.09.024
- D.B. Chithrani, Nanoparticles for improved therapeutics and imaging in cancer therapy. Recent Pat. Nanotechnol. 4(3), 171–180 (2010). doi:10.2174/187221010792483726
- A.G. Cuenca, H.B. Jiang, S.N. Hochwald, M. Delano, W.G. Cance, S.R. Grobmyer, Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer 107(3), 459–466 (2006). doi:10.1002/cncr.22035
- J. Rao, Shedding light on tumors using nanoparticles. ACS Nano 2(10), 1984–1986 (2008). doi:10.1021/nn800669n
- T.S. Hauck, T.L. Jennings, T. Yatsenko, J.C. Kumaradas, W.C.W. Chan, Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Adv. Mater. 20(20), 3832–3838 (2008). doi:10.1002/adma.200800921
- P. Alivisatos, The use of nanocrystals in biological detection. Nat. Biotechnol. 22, 47–51 (2003). doi:10.1038/nbt927
- M. Liong, J. Lu, M. Kovochich, T. Xia, S.G. Ruehm, A.E. Nel, F. Tamanoi, J.I. Zink, Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2(5), 889–896 (2008). doi:10.1021/nn800072t
- S. Langereis, J. Keupp, J.L.J. van Velthoven, I.H.C. de Roos, D. Burdinski, J.A. Pikkemaat, H. Grüll, J. Keupp, A temperature-sensitive liposomal 1H CEST and 19F contrast agent for mr image-guided drug delivery. JACS 131(4), 1380–1381 (2009). doi:10.1021/ja8087532
- S.D. Perrault, C. Walkey, T. Jennings, H.C. Fischer, W.C.W. Chan, Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 9(5), 1909–1915 (2009). doi:10.1021/nl900031y
- J.E. Lee, N. Lee, H. Kim, J. Kim, S.H. Choi, J.H. Kim, T. Kim, I.C. Song, S.P. Park, W.K. Moon, T. Hyeon, Uniform mesoporous dye-doped silica nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. JACS 132(2), 552–557 (2010). doi:10.1021/ja905793q
- S.D. Brown, P. Nativo, J.-A. Smith, D. Stirling, P.R. Edwards, B. Venugopal, D.J. Flint, J.A. Plumb, D. Graham, N.J. Wheate, Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. JACS 132(13), 4678–4684 (2010). doi:10.1021/ja908117a
- D.B. Chithrani, S. Jelveh, F. Jalali, M. van Prooijen, C. Allen, R.G. Bristow, R.P. Hill, D.A. Jaffray, Gold nanoparticles as a radiation sensitizer in cancer therapy. Radiat. Res. 173(6), 719–728 (2010). doi:10.1667/RR1984.1
- D. Yohan, B.D. Chithrani, Applications of nanoparticles in nanomedicine. J. Biomed. Nanotechnol. 10(9), 2371–2392 (2014). doi:10.1166/jbn.2014.2015
- H. Maeda, Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J. Control Release 164(2), 138–144 (2012). doi:10.1016/j.jconrel.2012.04.038
- K.O. Hicks, S.J. Ohms, P.L. van Zijl, W.A. Denny, P.J. Hunter, W.R. Wilson, An experimental and mathematical model for the extravascular transport of a DNA intercalator in tumours. Br. J. Cancer 76, 894–903 (1997). doi:10.1038/bjc.1997.481
- W.R. Wilson, K.O. Hicks, Measurement of extravascular drug diffusion in multicellular layers. Br. J. Cancer 79, 1623–6 (1999). Comment on P.M. Phillips, P.M. Loadman, B.P. Cronin, Evaluation of a novel in vitro assay for assessing drug penetration into avascular regions of tumors. Br. J. Cancer 77, 2112–9 (1998)
- I.F. Tannock, C.M. Lee, J.K. Tunggal, D.S. Cowan, M.J. Egorin, Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin. Cancer Res. 8(3), 878–884 (2002)
- D.S.K.O. Hicks Cowan, W.R. Wilson, Multicellular membranes as an in vitro model for extravascular diffusion in tumours. Br. J. Cancer Suppl. 27, S28–S31 (1996)
- A.I. Minchinton, K.R. Wendt, K.A. Clow, K.H. Fryer, Multilayers of cells growing on a permeable support. An in vitro tumour model. Acta Oncol. 36(1), 13–16 (1997). doi:10.3109/02841869709100724
- A.I. Minchinton, I.F. Tannock, Drug penetration in solid tumours. Nat. Rev. Cancer 6, 583–592 (2006). doi:10.1038/nrc1893
- R.H. Thomlinson, L.H. Gray, The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer 9, 539–549 (1955). doi:10.1038/bjc.1955.55
- I.F. Tannock, S. Hayashi, The proliferation of capillary endothelial cells. Cancer Res. 32(1), 77–82 (1972)
- J. Denekamp, B. Hobson, Endothelial-cell proliferation in experimental tumours. Br. J. Cancer 46, 711–720 (1982). doi:10.1038/bjc.1982.263
- P.A. Netti, D.A. Berk, M.A. Swartz, A.J. Grodzinsky, R.K. Jain Netti, Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 60(9), 2497–2503 (2000)
- CdeL Davies, D.A. Berk, A. Pluen, R.K. Jain, Comparison of IgG diffusion and extracellular matrix composition in rhabdomyosarcomas grown in mice versus in vitro as spheroids reveals the role of host stromal cells. Br. J. Cancer 86, 1639–1644 (2002). doi:10.1038/sj.bjc.6600270
- E. Brown, T. McKee, E. diTomaso, A. Pluen, B. Seed, Y. Boucher, R.K. Jain, Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat. Med. 9, 796–800 (2003). doi:10.1038/nm879
- G. Frens, Controlled nucleation for the regulation of particle size in monodisperse gold suspensions. Nat. Phys. Sci. 241, 20–22 (1973). doi:10.1038/physci241020a0
- G.S. Tennyson, B.P. Lane, In vivo and in vitro growth of a rat tracheal squamous cell carcinoma. Cancer Res. 41, 4687–4692 (1981)
- M.J. Evans, C.J. Kovacs, H.A. Hopkins, Properties of the H-4-II-E tumor cell system. II. In vitro characteristics of an experimental tumor cell line. Cell Prolif. 10, 245–254 (1977). doi:10.1111/j.1365-2184.1977.tb00292.x
- E.K. Rofstad, E.O. Pettersen, T. Lindmo, R. Oftebro, The proliferation kinetics of NHIK 1922 cells in vitro and in solid tumours in athymic mice. Cell Prolif. 13(2), 163–171 (1980). doi:10.1111/j.1365-2184.1980.tb00459.x
- K.O. Hicksa, Y. Fleminga, B.G. Siim, C.J. Koch, W.R. Wilson, Extravascular diffusion of tirapazamine: effect of metabolic consumption assessed using the multicellular layer model. Int. J. Radiat. Oncol. Biol. Phys. 42(3), 641–649 (1998). doi:10.1016/S0360-3016(98)00268-5
- C.J. Koch, J. Kruuv, H.E. Frey, R.A. Snyder, Plateau phase in growth induced by hypoxia. Int. J. Radiat. Biol. 23(1), 67–74 (1973). doi:10.1080/09553007314550061
- D.G. Hirst, J. Denekamp, Tumour cell proliferation in relation to the vasculature. Cell Prolif. 12(1), 31–42 (1979). doi:10.1111/j.1365-2184.1979.tb00111.x
- L.A. Liotta, U.P. Thorgeirsson, S. Garbisa, Role of collagenases in tumor cell invasion. Cancer Metastasis Rev. 1(4), 277–288 (1982). doi:10.1007/BF00124213
- G. Wirl, J. Frick, Collagenase–a marker enzyme in human bladder cancer? Urol. Res. 7(2), 103–108 (1979). doi:10.1007/BF00254689
- J. Gross, Y. Nagai, Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. PNAS ESI 54(4), 1197–1204 (1965). doi:10.1073/pnas.54.4.1197
- W.M. Saltzman, M.L. Radomsky, K.J. Whaley, R.A. Cone, Antibody diffusion in human cervical mucus. Biophys. J. 66(2), 508–515 (1994). doi:10.1016/S0006-3495(94)80802-1
- J.R. Levick, Flow through interstitium and other fibrous matrices. Q. J. Exp. Physiol. 72(4), 409–437 (1987)
- V.H. Barocas, R.T. Tranquillo, An anisotropic biphasic theory of tissue-equivalent mechanics: the interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. J. Biomech. Eng. 119(2), 137–145 (1997). doi:10.1115/1.2796072
- I.F. Tannock, C.M. Lee, J.K. Tunggal, D.S.M. Cowan, M.J. Egorin, Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin. Cancer Res. 8, 878–884 (2002)
- A.J. Primeau, A. Rendon, D. Hedley, L. Lilge, I.F. Tannock, The distribution of the anticancer drug Doxorubicin in relation to blood vessels in solid tumors. Clin. Cancer Res. 11, 8782–8788 (2005). doi:10.1158/1078-0432.CCR-05-1664
- I.F. Tannock, The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br. J. Cancer 22(2), 258–273 (1968). doi:10.1038/bjc.1968.34
- B.D. Chithrani, A.A. Ghazani, W.C.W. Chan, Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 6(4), 662–668 (2006). doi:10.1021/nl052396o
- B.D. Chithrani, W.C.W. Chan, Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 7(6), 1542–1550 (2007). doi:10.1021/nl070363y
- P. Puvanakrishnan, J. Park, D. Chatterjee, S. Krishnan, J.W. Tunnell, In vivo tumor targeting of gold nanoparticles: effect of particle type and dosing strategy. Int. J. Nanomed. 7, 1251–1258 (2012). doi:10.2147/IJN.S29147
- G. Zhang, Z. Yang, W. Lu, R. Zhang, Q. Huang, M. Tian, L. Li, D. Liang, C. Li, Influence of anchoring ligands and particle size on the colloidal stability band in vivo biodistribution of polyethylene glycol coated gold nanoparticles in tumor-xenografted mice. Biomaterials 30(10), 1928–1936 (2009). doi:10.1016/j.biomaterials.2008.12.038
References
J. Lee, L.D.K. Chatterjee, M.H. Lee, I. Krishnan, Gold nanoparticles in breast cancer treatment: promise and potential pitfalls. Cancer Lett. 347(1), 46–53 (2014). doi:10.1016/j.canlet.2014.02.006
B.D. Chithrani, Optimization of bio-nano interface using gold nanostructures as a model nanoparticle system. Insciences J. 1, 136–156 (2011)
J.-L. Li, W. Wang, X.-Y. Liu, Z.-P. Zhang, H.-C. Guo, W.-M. Liu, S.-H. Tang, In vitro cancer cell imaging and therapy using transferrin-conjugated gold nanoparticles. Cancer Lett. 274(2), 319–326 (2008). doi:10.1016/j.canlet.2008.09.024
D.B. Chithrani, Nanoparticles for improved therapeutics and imaging in cancer therapy. Recent Pat. Nanotechnol. 4(3), 171–180 (2010). doi:10.2174/187221010792483726
A.G. Cuenca, H.B. Jiang, S.N. Hochwald, M. Delano, W.G. Cance, S.R. Grobmyer, Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer 107(3), 459–466 (2006). doi:10.1002/cncr.22035
J. Rao, Shedding light on tumors using nanoparticles. ACS Nano 2(10), 1984–1986 (2008). doi:10.1021/nn800669n
T.S. Hauck, T.L. Jennings, T. Yatsenko, J.C. Kumaradas, W.C.W. Chan, Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Adv. Mater. 20(20), 3832–3838 (2008). doi:10.1002/adma.200800921
P. Alivisatos, The use of nanocrystals in biological detection. Nat. Biotechnol. 22, 47–51 (2003). doi:10.1038/nbt927
M. Liong, J. Lu, M. Kovochich, T. Xia, S.G. Ruehm, A.E. Nel, F. Tamanoi, J.I. Zink, Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2(5), 889–896 (2008). doi:10.1021/nn800072t
S. Langereis, J. Keupp, J.L.J. van Velthoven, I.H.C. de Roos, D. Burdinski, J.A. Pikkemaat, H. Grüll, J. Keupp, A temperature-sensitive liposomal 1H CEST and 19F contrast agent for mr image-guided drug delivery. JACS 131(4), 1380–1381 (2009). doi:10.1021/ja8087532
S.D. Perrault, C. Walkey, T. Jennings, H.C. Fischer, W.C.W. Chan, Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 9(5), 1909–1915 (2009). doi:10.1021/nl900031y
J.E. Lee, N. Lee, H. Kim, J. Kim, S.H. Choi, J.H. Kim, T. Kim, I.C. Song, S.P. Park, W.K. Moon, T. Hyeon, Uniform mesoporous dye-doped silica nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. JACS 132(2), 552–557 (2010). doi:10.1021/ja905793q
S.D. Brown, P. Nativo, J.-A. Smith, D. Stirling, P.R. Edwards, B. Venugopal, D.J. Flint, J.A. Plumb, D. Graham, N.J. Wheate, Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. JACS 132(13), 4678–4684 (2010). doi:10.1021/ja908117a
D.B. Chithrani, S. Jelveh, F. Jalali, M. van Prooijen, C. Allen, R.G. Bristow, R.P. Hill, D.A. Jaffray, Gold nanoparticles as a radiation sensitizer in cancer therapy. Radiat. Res. 173(6), 719–728 (2010). doi:10.1667/RR1984.1
D. Yohan, B.D. Chithrani, Applications of nanoparticles in nanomedicine. J. Biomed. Nanotechnol. 10(9), 2371–2392 (2014). doi:10.1166/jbn.2014.2015
H. Maeda, Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J. Control Release 164(2), 138–144 (2012). doi:10.1016/j.jconrel.2012.04.038
K.O. Hicks, S.J. Ohms, P.L. van Zijl, W.A. Denny, P.J. Hunter, W.R. Wilson, An experimental and mathematical model for the extravascular transport of a DNA intercalator in tumours. Br. J. Cancer 76, 894–903 (1997). doi:10.1038/bjc.1997.481
W.R. Wilson, K.O. Hicks, Measurement of extravascular drug diffusion in multicellular layers. Br. J. Cancer 79, 1623–6 (1999). Comment on P.M. Phillips, P.M. Loadman, B.P. Cronin, Evaluation of a novel in vitro assay for assessing drug penetration into avascular regions of tumors. Br. J. Cancer 77, 2112–9 (1998)
I.F. Tannock, C.M. Lee, J.K. Tunggal, D.S. Cowan, M.J. Egorin, Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin. Cancer Res. 8(3), 878–884 (2002)
D.S.K.O. Hicks Cowan, W.R. Wilson, Multicellular membranes as an in vitro model for extravascular diffusion in tumours. Br. J. Cancer Suppl. 27, S28–S31 (1996)
A.I. Minchinton, K.R. Wendt, K.A. Clow, K.H. Fryer, Multilayers of cells growing on a permeable support. An in vitro tumour model. Acta Oncol. 36(1), 13–16 (1997). doi:10.3109/02841869709100724
A.I. Minchinton, I.F. Tannock, Drug penetration in solid tumours. Nat. Rev. Cancer 6, 583–592 (2006). doi:10.1038/nrc1893
R.H. Thomlinson, L.H. Gray, The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer 9, 539–549 (1955). doi:10.1038/bjc.1955.55
I.F. Tannock, S. Hayashi, The proliferation of capillary endothelial cells. Cancer Res. 32(1), 77–82 (1972)
J. Denekamp, B. Hobson, Endothelial-cell proliferation in experimental tumours. Br. J. Cancer 46, 711–720 (1982). doi:10.1038/bjc.1982.263
P.A. Netti, D.A. Berk, M.A. Swartz, A.J. Grodzinsky, R.K. Jain Netti, Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 60(9), 2497–2503 (2000)
CdeL Davies, D.A. Berk, A. Pluen, R.K. Jain, Comparison of IgG diffusion and extracellular matrix composition in rhabdomyosarcomas grown in mice versus in vitro as spheroids reveals the role of host stromal cells. Br. J. Cancer 86, 1639–1644 (2002). doi:10.1038/sj.bjc.6600270
E. Brown, T. McKee, E. diTomaso, A. Pluen, B. Seed, Y. Boucher, R.K. Jain, Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat. Med. 9, 796–800 (2003). doi:10.1038/nm879
G. Frens, Controlled nucleation for the regulation of particle size in monodisperse gold suspensions. Nat. Phys. Sci. 241, 20–22 (1973). doi:10.1038/physci241020a0
G.S. Tennyson, B.P. Lane, In vivo and in vitro growth of a rat tracheal squamous cell carcinoma. Cancer Res. 41, 4687–4692 (1981)
M.J. Evans, C.J. Kovacs, H.A. Hopkins, Properties of the H-4-II-E tumor cell system. II. In vitro characteristics of an experimental tumor cell line. Cell Prolif. 10, 245–254 (1977). doi:10.1111/j.1365-2184.1977.tb00292.x
E.K. Rofstad, E.O. Pettersen, T. Lindmo, R. Oftebro, The proliferation kinetics of NHIK 1922 cells in vitro and in solid tumours in athymic mice. Cell Prolif. 13(2), 163–171 (1980). doi:10.1111/j.1365-2184.1980.tb00459.x
K.O. Hicksa, Y. Fleminga, B.G. Siim, C.J. Koch, W.R. Wilson, Extravascular diffusion of tirapazamine: effect of metabolic consumption assessed using the multicellular layer model. Int. J. Radiat. Oncol. Biol. Phys. 42(3), 641–649 (1998). doi:10.1016/S0360-3016(98)00268-5
C.J. Koch, J. Kruuv, H.E. Frey, R.A. Snyder, Plateau phase in growth induced by hypoxia. Int. J. Radiat. Biol. 23(1), 67–74 (1973). doi:10.1080/09553007314550061
D.G. Hirst, J. Denekamp, Tumour cell proliferation in relation to the vasculature. Cell Prolif. 12(1), 31–42 (1979). doi:10.1111/j.1365-2184.1979.tb00111.x
L.A. Liotta, U.P. Thorgeirsson, S. Garbisa, Role of collagenases in tumor cell invasion. Cancer Metastasis Rev. 1(4), 277–288 (1982). doi:10.1007/BF00124213
G. Wirl, J. Frick, Collagenase–a marker enzyme in human bladder cancer? Urol. Res. 7(2), 103–108 (1979). doi:10.1007/BF00254689
J. Gross, Y. Nagai, Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. PNAS ESI 54(4), 1197–1204 (1965). doi:10.1073/pnas.54.4.1197
W.M. Saltzman, M.L. Radomsky, K.J. Whaley, R.A. Cone, Antibody diffusion in human cervical mucus. Biophys. J. 66(2), 508–515 (1994). doi:10.1016/S0006-3495(94)80802-1
J.R. Levick, Flow through interstitium and other fibrous matrices. Q. J. Exp. Physiol. 72(4), 409–437 (1987)
V.H. Barocas, R.T. Tranquillo, An anisotropic biphasic theory of tissue-equivalent mechanics: the interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. J. Biomech. Eng. 119(2), 137–145 (1997). doi:10.1115/1.2796072
I.F. Tannock, C.M. Lee, J.K. Tunggal, D.S.M. Cowan, M.J. Egorin, Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin. Cancer Res. 8, 878–884 (2002)
A.J. Primeau, A. Rendon, D. Hedley, L. Lilge, I.F. Tannock, The distribution of the anticancer drug Doxorubicin in relation to blood vessels in solid tumors. Clin. Cancer Res. 11, 8782–8788 (2005). doi:10.1158/1078-0432.CCR-05-1664
I.F. Tannock, The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br. J. Cancer 22(2), 258–273 (1968). doi:10.1038/bjc.1968.34
B.D. Chithrani, A.A. Ghazani, W.C.W. Chan, Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 6(4), 662–668 (2006). doi:10.1021/nl052396o
B.D. Chithrani, W.C.W. Chan, Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 7(6), 1542–1550 (2007). doi:10.1021/nl070363y
P. Puvanakrishnan, J. Park, D. Chatterjee, S. Krishnan, J.W. Tunnell, In vivo tumor targeting of gold nanoparticles: effect of particle type and dosing strategy. Int. J. Nanomed. 7, 1251–1258 (2012). doi:10.2147/IJN.S29147
G. Zhang, Z. Yang, W. Lu, R. Zhang, Q. Huang, M. Tian, L. Li, D. Liang, C. Li, Influence of anchoring ligands and particle size on the colloidal stability band in vivo biodistribution of polyethylene glycol coated gold nanoparticles in tumor-xenografted mice. Biomaterials 30(10), 1928–1936 (2009). doi:10.1016/j.biomaterials.2008.12.038