Substitute of Expensive Pt with Improved Electrocatalytic Performance and Higher Resistance to CO Poisoning for Methanol Oxidation: the Case of Synergistic Pt-Co3O4 Nanocomposite
Corresponding Author: Zhi Zheng
Nano-Micro Letters,
Vol. 5 No. 4 (2013), Article Number: 296-302
Abstract
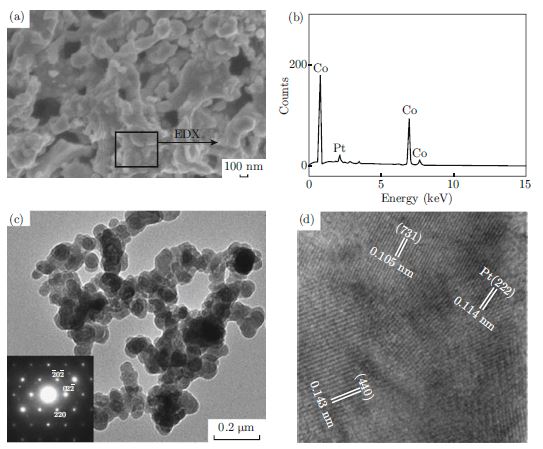
In this paper, Pt-Co3O4 nanocomposite was synthesized by a sol-gel process combined with electrodeposition method. Its electrocatalytic activity towards methanol oxidation was investigated at room temperature using cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and current density-time curve. It is found that the resultant Pt-Co3O4 catalysts with minute amount of Pt exhibite attractive electrocatalytic activity for methanol oxidation reaction (MOR) but with a high resistance CO poisoning due to the synergistic effects from Pt and Co3O4. Together with the low manufacturing cost of Co3O4, the reported nanostructured Pt-Co3O4 catalyst is expected to be a promising electrode material for direct methanol fuel cells (DMFC).
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- M. Islam, R. Basnayake and C. Korzeniewski, “A study of formaldehyde formation during methanol oxidation over PtRu bulk alloys and nanometer scale catalyst”, J. Electroanal. Chem. 599(1), 31–40 (2007). http://dx.doi.org/10.1016/j.jelechem.2006.08.010
- J. Q. Kang, W. T. Ma, J. J. Wu and M. Pan, “A novel catalyst Pt@NiPcTs/C: Synthesis, structural and electro-oxidation for methanol”, Catal. Commun. 10(8), 1271–1274 (2009). http://dx.doi.org/10.1016/j.catcom.2009.02.006
- Y. Zhang, M. Janyasupab, C. W. Liu, X. X. Li, J. Q. Xu and C. C. Liu, “Three dimensional PtRh alloy porous nanostructures: tuning the atomic composition and controlling the morphology for the application of direct methanol fuel cells”, Adv. Funct. Mater. 22(17), 3570–3575 (2012). http://dx.doi.org/10.1002/adfm.201200678
- J. Zeng, C. Francia, C. Gerbaldi, M. A. Dumitrescu, S. Specchia and P. Spinelli, “Smart synthesis of hollow core mesoporous shell carbons (HCMSC) as effective catalyst supports for methanol oxidation and oxygen reduction reactions”, J. Solid Electrochem. 16(9), 3087–3096 (2012). http://dx.doi.org/10.1007/s10008-012-1750-3
- Y. C. Zhao, X. L. Yang, J. N. Tian, F. Y. Wang and L. Zhan, “Methanol electro-oxidation on Ni@Pd core-shell nanoparticles supported on multi-walled carbon nanotubes in alkaline media”, Int. J. Hydrogen Energ. 35(8), 3249–3257 (2010). http://dx.doi.org/10.1016/j.ijhydene.2010.01.112
- J. Rossmeisl, P. Ferrin, Tritsaris and A. Georgios, “Bifunctional anode catalysts for direct methanol fuel cells”, Energ. Environ. Sci. 5(8), 8335–8342 (2012). http://dx.doi.org/10.1039/c2ee21455e
- T. Tomai, Y. Kawaguchi, S. Mitani and I. Honma, “Pt sub-nano/nanoclusters stabilized at the edge of nanographene sheets and their catalytic performance”, Electrochim. Acta 92, 421–426 (2013). http://dx.doi.org/10.1016/j.electacta.2013.01.067
- S. A. Tenney, B. A. Cagg, M. S. Levine, W. He, K. Manandhar and D. A. Chen, “Enhanced activity for supported Au clusters: methanol oxidation on Au/TiO2(110)”, Sur. Sci. 606(15–16), 1233–1243 (2012). http://dx.doi.org/10.1016/j.susc.2012.04.002
- J. Z. Sun, Y. Z. Wang, C. Zhang, T. Y. Kou and Z. H. Zhang, “Anodization driven enhancement of catalytic activity of Pd towards electro-oxidation of methanol, ethanol and formic acid”, Electrochem. Commun. 21, 42–45 (2012). http://dx.doi.org/10.1016/j.elecom.2012.04.023
- G. Z. Hu, F. Nitze, H. R. Barzegar, T. Sharifi, A. Mikołajczuk, C. W. Tai, A. Borodzinski and T. Wåberg, “Palladium nanocrystals supported on helical carbon nano?bers for highly ef?cient electro-oxidation of formic acid, methanol and ethanol in alkaline electrolytes”, J. Power Sources 209, 236–242 (2012). http://dx.doi.org/10.1016/j.jpowsour.2012.02.080
- I. Palacio, J. M. Rojo and O. Rodriguez de la Fuente, “Surface defects activating new reaction paths: formation of formate during methanol oxidation on Ru(0001)”, ChemPhysChem 13(9), 2354–2360 (2012). http://dx.doi.org/10.1002/cphc.201200190
- Y. Zhao, Y. Zhou, R. O’Hayre and Z. Shao, “Electrocatalytic oxidation of methanol on Pt catalyst supported on nitrogen-doped graphene induced by hydrazine reduction”, J. Phys. Chem. Solids 74(11), 1608–1614 (2013). http://dx.doi.org/10.1016/j.jpcs.2013.06.004
- Y. T. Liu, Q. B. Yuan, D. H. Duan, Z. L. Zhang, X. G. Hao, G. Q. Wei and S. B. Liu, “Electrochemical activity and stability of core—shell Fe2O3/Pt nanoparticles for methanol oxidatio”, J. Power Sources 243, 622–629 (2013). http://dx.doi.org/10.1016/j.jpowsour.2013.06.029
- A. B. A. A. Nassr, I. Sinev, W. Grünert and M. Bron, “PtNi supported on oxygen functionalized carbon nanotubes: In depth structural characterization and activity for methanol electrooxidation”, Appl. Catal. B: Envir. 142–143, 849–860 (2013). http://dx.doi.org/10.1016/j.apcatb.2013.06.013
- M. Takahashi, T. Mori, F. Ye and A. Vinu, “A study of formaldehyde formation during methanol oxidation over PtRu bulk alloys and nanometer scale catalyst”, J. Am. Ceram. Soc. 90(4), 1291–1294 (2007). http://dx.doi.org/10.1111/j.1551-2916.2006.01483.x
- X. L. Yang, X. Y. Wang, G. Q. Zhang, J. P. Zheng, T. S. Wang, X. Z. Liu, C. Y. Shu, L. Jiang and C. N. Wang, “Enhanced electrocatalytic performance for methanol oxidation of Pt nanoparticles on Mn3O4-modifiedmulti-walled carbon nanotubes”, Int. J. Hydrogen Energ. 37(15), 11167–11175 (2012). http://dx.doi.org/10.1016/j.ijhydene.2012.04.153
- X. W. Xie, Y. Li, Z. Q. Liu, M. Haruta and W. J. Shen, “Low-temperature oxidation of CO catalysed by Co3O4 nanorods”, Nature 458, 746–749 (2009). http://dx.doi.org/10.1038/nature07877
- C. Liu, Q. Liu, L. Bai, A. Dong, G. Liu and S. Wen, “Structure and catalytic performances of nanocrystalline Co3O4 catalysts for low temperature CO oxidation prepared by dry and wet synthetic routes”, J. Molecular Catal. A: Chem. 370, 1–6 (2013). http://dx.doi.org/10.1016/j.molcata.2012.12.003
- G. Marbán, I. López, T. Valdés-Solís and A. B. Fuertes, “Highly active structured catalyst made up of mesoporous Co3O4 nanowires supported on a metal wire mesh for the preferential oxidation of CO”, Int. J. Hydrogen Energ. 33(22), 6687–6695 (2008). http://dx.doi.org/10.1016/j.ijhydene.2008.07.067
- C. W. Xu, Z. Q. Tian, P. K. Shen and S. P. Jiang, “Oxide (CeO2, NiO, Co3O4 and Mn3O4)-promoted Pd/C electrocatalysts for alcohol electrooxidation in alkaline media”, Electrochim. Acta 53(5), 2610–2618 (2008). http://dx.doi.org/10.1016/j.electacta.2007.10.036
- Y. Y. Huang, J. D. Cai, S. Y. Zheng and Y. L. Guo, “Fabrication of a high-performance Pb-PtCu/CNT catalyst for methanol electro-oxidation”, J. Power Sources 210, 81–85 (2012). http://dx.doi.org/10.1016/j.jpowsour.2012.03.002
- S. W. Li, X. L. Yu, G. J. Zhang, Y. Ma and J. N. Yao, “Green synthesis of a Pt nanoparticle/polyoxometalate/carbon nanotube tri-component hybrid and its activity in the electrocatalysis of methanol oxidation”, Carbon 49(6), 1906–1911 (2011). http://dx.doi.org/10.1016/j.carbon.2011.01.015
- R. J. Liu, S. W. Li, X. L. Yu, G. J. Zhang, S. J. Zhang, J. N. Yao and L. J. Zhi, “A general green strategy for fabricating metal nanoparticles/polyoxometalate/graphene tri-component nanohybrids: enhanced electrocatalytic properties”, J. Mater. Chem. 22, 3319–3322 (2012). http://dx.doi.org/10.1039/c2jm15875b
- C. Z. Yang, N. K. van der Laak, K. Y. Chan and X. Zhang, “Microwave-assisted microemulsion synthesis of carbon supported Pt-WO3 nanoparticles as an electrocatalyst for methanol oxidation”, Electrochim. Acta 75, 262–272 (2012). http://dx.doi.org/10.1016/j.electacta.2012.04.107
- C. Lamy and E. M. Belgsir, J. M. Léger, “Electrocatalytic oxidation of aliphatic alcohols: Application to the direct alcohol fuel cell (DAFC)”, J. Appl. Electrochem. 31(7), 799–809 (2001). http://dx.doi.org/10.1023/A:1017587310150
- J. M. pérez, B. Beden, F. Hahn, A. Aldaz, C. Lamy, “In situ” infrared reflectance spectroscopic study of the early stages of ethanol adsorption at a platinum electrode in acid medium”, J. Electronanal. Chem. 262(1–2), 251–261 (1989). http://dx.doi.org/10.1016/0022-0728(89)80026-9
- H. Hitmi, E. M. Belgsir, J. M. Léger, C. Lamy and R. O. Lezna, “A kinetic analysis of the electro-oxidation of ethanol at a platinum electrode in acid medium”, Electrochim. Acta 39(3), 407–415 (1994). http://dx.doi.org/10.1016/0013-4686(94)80080-4
- J. Jansson, “Low-Temperature CO Oxidation over Co3O4/Al2O3”, J. Catal. 194(1), 55–60 (2000). http://dx.doi.org/10.1006/jcat.2000.2924
- Z. L. Zhang, H. R. Geng, L. S. Zheng and B. Du, “Characterization and catalytic activity for the NO decomposition and reduction by CO of nanosized Co3O4”, J. Alloy. Compd. 392(1–2), 317–321 (2005). http://dx.doi.org/10.1016/j.jallcom.2004.09.013
References
M. Islam, R. Basnayake and C. Korzeniewski, “A study of formaldehyde formation during methanol oxidation over PtRu bulk alloys and nanometer scale catalyst”, J. Electroanal. Chem. 599(1), 31–40 (2007). http://dx.doi.org/10.1016/j.jelechem.2006.08.010
J. Q. Kang, W. T. Ma, J. J. Wu and M. Pan, “A novel catalyst Pt@NiPcTs/C: Synthesis, structural and electro-oxidation for methanol”, Catal. Commun. 10(8), 1271–1274 (2009). http://dx.doi.org/10.1016/j.catcom.2009.02.006
Y. Zhang, M. Janyasupab, C. W. Liu, X. X. Li, J. Q. Xu and C. C. Liu, “Three dimensional PtRh alloy porous nanostructures: tuning the atomic composition and controlling the morphology for the application of direct methanol fuel cells”, Adv. Funct. Mater. 22(17), 3570–3575 (2012). http://dx.doi.org/10.1002/adfm.201200678
J. Zeng, C. Francia, C. Gerbaldi, M. A. Dumitrescu, S. Specchia and P. Spinelli, “Smart synthesis of hollow core mesoporous shell carbons (HCMSC) as effective catalyst supports for methanol oxidation and oxygen reduction reactions”, J. Solid Electrochem. 16(9), 3087–3096 (2012). http://dx.doi.org/10.1007/s10008-012-1750-3
Y. C. Zhao, X. L. Yang, J. N. Tian, F. Y. Wang and L. Zhan, “Methanol electro-oxidation on Ni@Pd core-shell nanoparticles supported on multi-walled carbon nanotubes in alkaline media”, Int. J. Hydrogen Energ. 35(8), 3249–3257 (2010). http://dx.doi.org/10.1016/j.ijhydene.2010.01.112
J. Rossmeisl, P. Ferrin, Tritsaris and A. Georgios, “Bifunctional anode catalysts for direct methanol fuel cells”, Energ. Environ. Sci. 5(8), 8335–8342 (2012). http://dx.doi.org/10.1039/c2ee21455e
T. Tomai, Y. Kawaguchi, S. Mitani and I. Honma, “Pt sub-nano/nanoclusters stabilized at the edge of nanographene sheets and their catalytic performance”, Electrochim. Acta 92, 421–426 (2013). http://dx.doi.org/10.1016/j.electacta.2013.01.067
S. A. Tenney, B. A. Cagg, M. S. Levine, W. He, K. Manandhar and D. A. Chen, “Enhanced activity for supported Au clusters: methanol oxidation on Au/TiO2(110)”, Sur. Sci. 606(15–16), 1233–1243 (2012). http://dx.doi.org/10.1016/j.susc.2012.04.002
J. Z. Sun, Y. Z. Wang, C. Zhang, T. Y. Kou and Z. H. Zhang, “Anodization driven enhancement of catalytic activity of Pd towards electro-oxidation of methanol, ethanol and formic acid”, Electrochem. Commun. 21, 42–45 (2012). http://dx.doi.org/10.1016/j.elecom.2012.04.023
G. Z. Hu, F. Nitze, H. R. Barzegar, T. Sharifi, A. Mikołajczuk, C. W. Tai, A. Borodzinski and T. Wåberg, “Palladium nanocrystals supported on helical carbon nano?bers for highly ef?cient electro-oxidation of formic acid, methanol and ethanol in alkaline electrolytes”, J. Power Sources 209, 236–242 (2012). http://dx.doi.org/10.1016/j.jpowsour.2012.02.080
I. Palacio, J. M. Rojo and O. Rodriguez de la Fuente, “Surface defects activating new reaction paths: formation of formate during methanol oxidation on Ru(0001)”, ChemPhysChem 13(9), 2354–2360 (2012). http://dx.doi.org/10.1002/cphc.201200190
Y. Zhao, Y. Zhou, R. O’Hayre and Z. Shao, “Electrocatalytic oxidation of methanol on Pt catalyst supported on nitrogen-doped graphene induced by hydrazine reduction”, J. Phys. Chem. Solids 74(11), 1608–1614 (2013). http://dx.doi.org/10.1016/j.jpcs.2013.06.004
Y. T. Liu, Q. B. Yuan, D. H. Duan, Z. L. Zhang, X. G. Hao, G. Q. Wei and S. B. Liu, “Electrochemical activity and stability of core—shell Fe2O3/Pt nanoparticles for methanol oxidatio”, J. Power Sources 243, 622–629 (2013). http://dx.doi.org/10.1016/j.jpowsour.2013.06.029
A. B. A. A. Nassr, I. Sinev, W. Grünert and M. Bron, “PtNi supported on oxygen functionalized carbon nanotubes: In depth structural characterization and activity for methanol electrooxidation”, Appl. Catal. B: Envir. 142–143, 849–860 (2013). http://dx.doi.org/10.1016/j.apcatb.2013.06.013
M. Takahashi, T. Mori, F. Ye and A. Vinu, “A study of formaldehyde formation during methanol oxidation over PtRu bulk alloys and nanometer scale catalyst”, J. Am. Ceram. Soc. 90(4), 1291–1294 (2007). http://dx.doi.org/10.1111/j.1551-2916.2006.01483.x
X. L. Yang, X. Y. Wang, G. Q. Zhang, J. P. Zheng, T. S. Wang, X. Z. Liu, C. Y. Shu, L. Jiang and C. N. Wang, “Enhanced electrocatalytic performance for methanol oxidation of Pt nanoparticles on Mn3O4-modifiedmulti-walled carbon nanotubes”, Int. J. Hydrogen Energ. 37(15), 11167–11175 (2012). http://dx.doi.org/10.1016/j.ijhydene.2012.04.153
X. W. Xie, Y. Li, Z. Q. Liu, M. Haruta and W. J. Shen, “Low-temperature oxidation of CO catalysed by Co3O4 nanorods”, Nature 458, 746–749 (2009). http://dx.doi.org/10.1038/nature07877
C. Liu, Q. Liu, L. Bai, A. Dong, G. Liu and S. Wen, “Structure and catalytic performances of nanocrystalline Co3O4 catalysts for low temperature CO oxidation prepared by dry and wet synthetic routes”, J. Molecular Catal. A: Chem. 370, 1–6 (2013). http://dx.doi.org/10.1016/j.molcata.2012.12.003
G. Marbán, I. López, T. Valdés-Solís and A. B. Fuertes, “Highly active structured catalyst made up of mesoporous Co3O4 nanowires supported on a metal wire mesh for the preferential oxidation of CO”, Int. J. Hydrogen Energ. 33(22), 6687–6695 (2008). http://dx.doi.org/10.1016/j.ijhydene.2008.07.067
C. W. Xu, Z. Q. Tian, P. K. Shen and S. P. Jiang, “Oxide (CeO2, NiO, Co3O4 and Mn3O4)-promoted Pd/C electrocatalysts for alcohol electrooxidation in alkaline media”, Electrochim. Acta 53(5), 2610–2618 (2008). http://dx.doi.org/10.1016/j.electacta.2007.10.036
Y. Y. Huang, J. D. Cai, S. Y. Zheng and Y. L. Guo, “Fabrication of a high-performance Pb-PtCu/CNT catalyst for methanol electro-oxidation”, J. Power Sources 210, 81–85 (2012). http://dx.doi.org/10.1016/j.jpowsour.2012.03.002
S. W. Li, X. L. Yu, G. J. Zhang, Y. Ma and J. N. Yao, “Green synthesis of a Pt nanoparticle/polyoxometalate/carbon nanotube tri-component hybrid and its activity in the electrocatalysis of methanol oxidation”, Carbon 49(6), 1906–1911 (2011). http://dx.doi.org/10.1016/j.carbon.2011.01.015
R. J. Liu, S. W. Li, X. L. Yu, G. J. Zhang, S. J. Zhang, J. N. Yao and L. J. Zhi, “A general green strategy for fabricating metal nanoparticles/polyoxometalate/graphene tri-component nanohybrids: enhanced electrocatalytic properties”, J. Mater. Chem. 22, 3319–3322 (2012). http://dx.doi.org/10.1039/c2jm15875b
C. Z. Yang, N. K. van der Laak, K. Y. Chan and X. Zhang, “Microwave-assisted microemulsion synthesis of carbon supported Pt-WO3 nanoparticles as an electrocatalyst for methanol oxidation”, Electrochim. Acta 75, 262–272 (2012). http://dx.doi.org/10.1016/j.electacta.2012.04.107
C. Lamy and E. M. Belgsir, J. M. Léger, “Electrocatalytic oxidation of aliphatic alcohols: Application to the direct alcohol fuel cell (DAFC)”, J. Appl. Electrochem. 31(7), 799–809 (2001). http://dx.doi.org/10.1023/A:1017587310150
J. M. pérez, B. Beden, F. Hahn, A. Aldaz, C. Lamy, “In situ” infrared reflectance spectroscopic study of the early stages of ethanol adsorption at a platinum electrode in acid medium”, J. Electronanal. Chem. 262(1–2), 251–261 (1989). http://dx.doi.org/10.1016/0022-0728(89)80026-9
H. Hitmi, E. M. Belgsir, J. M. Léger, C. Lamy and R. O. Lezna, “A kinetic analysis of the electro-oxidation of ethanol at a platinum electrode in acid medium”, Electrochim. Acta 39(3), 407–415 (1994). http://dx.doi.org/10.1016/0013-4686(94)80080-4
J. Jansson, “Low-Temperature CO Oxidation over Co3O4/Al2O3”, J. Catal. 194(1), 55–60 (2000). http://dx.doi.org/10.1006/jcat.2000.2924
Z. L. Zhang, H. R. Geng, L. S. Zheng and B. Du, “Characterization and catalytic activity for the NO decomposition and reduction by CO of nanosized Co3O4”, J. Alloy. Compd. 392(1–2), 317–321 (2005). http://dx.doi.org/10.1016/j.jallcom.2004.09.013