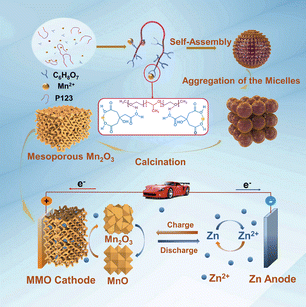
Boosting High-Rate Zinc-Storage Performance by the Rational Design of Mn2O3 Nanoporous Architecture Cathode
Corresponding Author: Zhen‑An Qiao
Nano-Micro Letters,
Vol. 12 (2020), Article Number: 14
Abstract
Manganese oxides are regarded as one of the most promising cathode materials in rechargeable aqueous Zn-ion batteries (ZIBs) because of the low price and high security. However, the practical application of Mn2O3 in ZIBs is still plagued by the low specific capacity and poor rate capability. Herein, highly crystalline Mn2O3 materials with interconnected mesostructures and controllable pore sizes are obtained via a ligand-assisted self-assembly process and used as high-performance electrode materials for reversible aqueous ZIBs. The coordination degree between Mn2+ and citric acid ligand plays a crucial role in the formation of the mesostructure, and the pore sizes can be easily tuned from 3.2 to 7.3 nm. Ascribed to the unique feature of nanoporous architectures, excellent zinc-storage performance can be achieved in ZIBs during charge/discharge processes. The Mn2O3 electrode exhibits high reversible capacity (233 mAh g−1 at 0.3 A g−1), superior rate capability (162 mAh g−1 retains at 3.08 A g−1) and remarkable cycling durability over 3000 cycles at a high current rate of 3.08 A g−1. Moreover, the corresponding electrode reaction mechanism is studied in depth according to a series of analytical methods. These results suggest that rational design of the nanoporous architecture for electrode materials can effectively improve the battery performance.
Highlights:
1 Highly crystalline Mn2O3 materials with tunable pore sizes are obtained and employed as high-performance cathode materials for reversible aqueous Zn-ion battery.
2 The Zn/Mn2O3 battery exhibits significantly improved rate capability and remarkable cycling durability due to the introduction of nanoporous architecture.
3 The Zn2+/H+ intercalations mechanism is put forward for the Zn/Mn2O3 battery.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- H. Chen, M. Ling, L. Hencz, H.Y. Ling, G. Li, Z. Lin, G. Liu, S. Zhang, Exploring chemical, mechanical, and electrical functionalities of binders for advanced energy-storage devices. Chem. Rev. 118(18), 8936–8982 (2018). https://doi.org/10.1021/acs.chemrev.8b00241
- Z.P. Cano, D. Banham, S. Ye, A. Hintennach, J. Lu, M. Fowler, Z. Chen, Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 3(4), 279–289 (2018). https://doi.org/10.1038/s41560-018-0108-1
- J. Mei, T. Liao, L. Kou, Z. Sun, Two-dimensional metal oxide nanomaterials for next-generation rechargeable batteries. Adv. Mater. 29(48), 1700176 (2017). https://doi.org/10.1002/adma.201700176
- Z.W. Chang, J.J. Xu, X.B. Zhang, Recent progress in electrocatalyst for Li-O2 batteries. Adv. Energy Mater. 7(23), 1700875 (2017). https://doi.org/10.1002/aenm.201700875
- M. Winter, B. Barnett, K. Xu, Before Li ion batteries. Chem. Rev. 118(23), 11433–11456 (2018). https://doi.org/10.1021/acs.chemrev.8b00422
- F.A. Susai, H. Sclar, Y. Shilina, T.R. Penki, R. Raman et al., Horizons for Li-ion batteries relevant to electro-mobility: high-specific-energy cathodes and chemically active separators. Adv. Mater. 30(41), 1801348 (2018). https://doi.org/10.1002/adma.201801348
- J.-M. Tarascon, M. Armand, Issues and challenges facing rechargeable lithium batteries. Nature 414(6861), 359–367 (2001). https://doi.org/10.1038/35104644
- C. Zhan, T. Wu, J. Lu, K. Amine, Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes: a critical review. Energy Environ. Sci. 11(2), 243–257 (2018). https://doi.org/10.1039/C7EE03122J
- W. Zhou, S. Wang, Y. Li, S. Xin, A. Manthiram, J.B. Goodenough, Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte. J. Am. Chem. Soc. 138(30), 9385–9388 (2016). https://doi.org/10.1021/jacs.6b05341
- L. Shen, L. Yu, X.Y. Yu, X. Zhang, X.W. Lou, Self-templated formation of uniform NiCo2O4 hollow spheres with complex interior structures for lithium-ion batteries and supercapacitors. Angew. Chem. Int. Ed. 54(6), 1868–1872 (2015). https://doi.org/10.1002/anie.201409776
- P.K. Nayak, L. Yang, W. Brehm, P. Adelhelm, From lithium-ion to sodium-ion batteries: advantages, challenges, and surprises. Angew. Chem. Int. Ed. 57(1), 102–120 (2018). https://doi.org/10.1002/ange.201703772
- Z. Zhang, S. Dong, Z. Cui, A. Du, G. Li, G. Cui, Rechargeable magnesium batteries using conversion-type cathodes: a perspective and minireview. Small Methods 2(10), 1800020 (2018). https://doi.org/10.1002/smtd.201800020
- X. Yu, B. Wang, D. Gong, Z. Xu, B. Lu, Graphene nanoribbons on highly porous 3D graphene for high-capacity and ultrastable Al-ion batteries. Adv. Mater. 29(4), 1604118 (2017). https://doi.org/10.1002/adma.201604118
- M. Song, H. Tan, D. Chao, H.J. Fan, Recent advances in Zn-ion batteries. Adv. Funct. Mater. 28(41), 1802564 (2018). https://doi.org/10.1002/adfm.201802564
- H. Li, L. McRae, C.J. Firby, A.Y. Elezzabi, Rechargeable aqueous electrochromic batteries utilizing Ti-substituted tungsten molybdenum oxide based Zn2+ ion intercalation cathodes. Adv. Mater. 31(15), 1807065 (2019). https://doi.org/10.1002/adma.201807065
- W. Xu, Y. Wang, Recent progress on zinc-ion rechargeable batteries. Nano-Micro Lett. 11, 90 (2019). https://doi.org/10.1007/s40820-019-0322-9
- Y.R. Qi, Y.X. Lu, F.X. Ding, Q.Q. Zhang, H. Li, J. Huang, L.Q. Chen, Y.S. Hu, Slope-dominated carbon anode with high specific capacity and superior rate capability for high safety Na-ion batteries. Angew. Chem. Int. Ed. 58(13), 4361–4365 (2019). https://doi.org/10.1002/anie.201900005
- Q. Liang, F. Chen, S. Wang, Q. Ru, Q. He, X. Hou, C.Y. Su, Y. Shi, An organic flow desalination battery. Energy Storage Mater. 20, 203–207 (2019). https://doi.org/10.1016/j.ensm.2018.11.006
- H. Pan, Y. Shao, P. Yan, Y. Cheng, K.S. Han et al., Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 1, 16039 (2016). https://doi.org/10.1038/nenergy.2016.39
- N. Zhang, F. Cheng, Y. Liu, Q. Zhao, K. Lei, C. Chen, X. Liu, J. Chen, Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J. Am. Chem. Soc. 138(39), 12894–12901 (2016). https://doi.org/10.1021/jacs.6b05958
- N. Zhang, F. Cheng, J. Liu, L. Wang, X. Long, X. Liu, F. Li, J. Chen, Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 8, 405 (2017). https://doi.org/10.1038/s41467-017-00467-x
- T. Luo, S. Wang, X. Hou, F. Chen, X. Liu, Z. Shao, H. Yan, Cr–Zn redox battery with NiFe2O4 as catalyst for enhanced degradation of Cr(VI) pollution. ACS Sustain. Chem. Eng. 7(1), 111–116 (2019). https://doi.org/10.1021/acssuschemeng.8b05299
- R. Trócoli, G. Kasiri, F. La Mantia, Phase transformation of copper hexacyanoferrate (KCuFe(CN)6) during zinc insertion: effect of co-ion intercalation. J. Power Sources 400, 167–171 (2018). https://doi.org/10.1016/j.jpowsour.2018.08.015
- G. Li, Z. Yang, Y. Jiang, C. Jin, W. Huang, X. Ding, Y. Huang, Towards polyvalent ion batteries: a zinc-ion battery based on NASICON structured Na3V2(PO4)3. Nano Energy 25, 211–217 (2016). https://doi.org/10.1016/j.nanoen.2016.04.051
- L. Shan, J. Zhou, M. Han, G. Fang, X. Cao, X. Wu, S. Liang, Reversible Zn-driven reduction displacement reaction in aqueous zinc-ion battery. J. Mater. Chem. A 7(13), 7355–7359 (2019). https://doi.org/10.1039/C9TA00125E
- X. Hou, Q. Liang, X. Hu, Y. Zhou, Q. Ru, F. Chen, S. Hu, Coupling desalination and energy storage with redox flow electrodes. Nanoscale 10(26), 12308–12314 (2018). https://doi.org/10.1039/C8NR02737D
- B. Jiang, C. Xu, C. Wu, L. Dong, J. Li, F. Kang, Manganese sesquioxide as cathode material for multivalent zinc ion battery with high capacity and long cycle life. Electrochim. Acta 229, 422–428 (2017). https://doi.org/10.1016/j.electacta.2017.01.163
- Y. Liu, X. Zhou, R. Liu, X. Li, Y. Bai, H. Xiao, Y. Wang, G. Yuan, Tailoring three-dimensional composite architecture for advanced zinc-ion batteries. ACS Appl. Mater. Interfaces 11(21), 19191–19199 (2019). https://doi.org/10.1021/acsami.9b04583
- F. Cheng, H. Wang, Z. Zhu, Y. Wang, T. Zhang, Z. Tao, J. Chen, Porous LiMn2O4 nanorods with durable high-rate capability for rechargeable Li-ion batteries. Energy Environ. Sci. 4(9), 3668–3675 (2011). https://doi.org/10.1039/C1EE01795K
- D. Wang, D. Choi, Z. Yang, V.V. Viswanathan, Z. Nie et al., Synthesis and Li-ion insertion properties of highly crystalline mesoporous rutile TiO2. Chem. Mater. 20(10), 3435–3442 (2008). https://doi.org/10.1021/cm8002589
- X. Li, G. Wu, X. Liu, W. Li, M. Li, Orderly integration of porous TiO2(B) nanosheets into bunchy hierarchical structure for high-rate and ultralong-lifespan lithium-ion batteries. Nano Energy 31, 1–8 (2017). https://doi.org/10.1016/j.nanoen.2016.11.002
- A.S. Poyraz, C.H. Kuo, S. Biswas, C.K. King’ondu, S.L. Suib, A general approach to crystalline and monomodal pore size mesoporous materials. Nat. Commun. 4, 2952 (2013). https://doi.org/10.1038/ncomms3952
- D. Feng, T.-N. Gao, M. Fan, A. Li, K. Li, T. Wang, Q. Huo, Z.-A. Qiao, A general ligand-assisted self-assembly approach to crystalline mesoporous metal oxides. NPG Asia Mater. 10, 800–809 (2018). https://doi.org/10.1038/s41427-018-0072-z
- L. Zhang, H. Dai, Y. Xia, H. Jiang, H. Zhang, H. He, Ultrasound-assisted nanocasting fabrication of ordered mesoporous MnO2 and Co3O4 with high surface areas and polycrystalline walls. J. Phys. Chem. C 114(6), 2694–2700 (2010). https://doi.org/10.1021/jp910159b
- X. Zhou, Y. Zhu, W. Luo, Y. Ren, P. Xu et al., Chelation-assisted soft-template synthesis of ordered mesoporous zinc oxides for low concentration gas sensing. J. Mater. Chem. A 4(39), 15064–15071 (2016). https://doi.org/10.1039/c6ta05687c
- P. Kar, S. Sardar, S. Ghosh, M.R. Parida, B. Liu, O.F. Mohammed, P. Lemmens, S.K. Pal, Nano surface engineering of Mn2O3 for potential light-harvesting application. J. Mater. Chem. C 3(31), 8200–8211 (2015). https://doi.org/10.1039/C5TC01475A
- M.S. Kolathodi, S.N. Hanumantha Rao, T.S. Natarajan, G. Singh, Beaded manganese oxide (Mn2O3) nanofibers: preparation and application for capacitive energy storage. J. Mater. Chem. A 4(20), 7883–7891 (2016). https://doi.org/10.1039/C6TA01948J
- D. Ji, H. Zhou, J. Zhang, Y. Dan, H. Yang, A. Yuan, Facile synthesis of a metal–organic framework-derived Mn2O3 nanowire coated three-dimensional graphene network for high-performance freestanding supercapacitor electrodes. J. Mater. Chem. A 4(21), 8283–8290 (2016). https://doi.org/10.1039/c6ta01377e
- K. Möller, J. Kobler, T. Bein, Colloidal suspensions of nanometer-sized mesoporous silica. Adv. Funct. Mater. 17(4), 605–612 (2007). https://doi.org/10.1002/adfm.200600578
- S.D. Han, S. Kim, D. Li, V. Petkov, H.D. Yoo et al., Mechanism of Zn insertion into nanostructured δ-MnO2: a nonaqueous rechargeable Zn metal battery. Chem. Mater. 29(11), 4874–4884 (2017). https://doi.org/10.1021/acs.chemmater.7b00852
- S. Islam, M.H. Alfaruqi, V. Mathew, J. Song, S. Kim et al., Facile synthesis and the exploration of the zinc storage mechanism of β-MnO2 nanorods with exposed (101) planes as a novel cathode material for high performance eco-friendly zinc-ion batteries. J. Mater. Chem. A 5(44), 23299–23309 (2017). https://doi.org/10.1039/c7ta07170a
- W. Sun, F. Wang, S. Hou, C. Yang, X. Fan et al., Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J. Am. Chem. Soc. 139(29), 9775–9778 (2017). https://doi.org/10.1021/jacs.7b04471
- B. Tang, G. Fang, J. Zhou, L. Wang, Y. Lei et al., Potassium vanadates with stable structure and fast ion diffusion channel as cathode for rechargeable aqueous zinc-ion batteries. Nano Energy 51, 579–587 (2018). https://doi.org/10.1016/j.nanoen.2018.07.014
- L. Ma, S. Chen, H. Li, Z. Ruan, Z. Tang et al., Initiating a mild aqueous electrolyte Co3O4/Zn battery with 2.2 V-high voltage and 5000-cycle lifespan by a Co(III) rich-electrode. Energy Environ. Sci. 11(9), 2521–2530 (2018). https://doi.org/10.1039/C8EE01415A
- Z. Guo, Y. Ma, X. Dong, J. Huang, Y. Wang, Y. Xia, An environmentally friendly and flexible aqueous zinc battery using an organic cathode. Angew. Chem. Int. Ed. 57(36), 11737–11741 (2018). https://doi.org/10.1002/anie.201807121
- C. Pan, R.G. Nuzzo, A.A. Gewirth, ZnAlxCo2−xO4 spinels as cathode materials for non-aqueous Zn batteries with an open circuit voltage of ≤ 2 V. Chem. Mater. 29(21), 9351–9359 (2017). https://doi.org/10.1021/acs.chemmater.7b03340
- B. Sambandam, V. Soundharrajan, S. Kim, M.H. Alfaruqi, J. Jo et al., Aqueous rechargeable Zn-ion batteries: an imperishable and high-energy Zn2V2O7 nanowire cathode through intercalation regulation. J. Mater. Chem. A 6(9), 3850–3856 (2018). https://doi.org/10.1039/c7ta11237h
- W. Li, K. Wang, S. Cheng, K. Jiang, A long-life aqueous Zn-ion battery based on Na3V2(PO4)2F3 cathode. Energy Storage Mater. 15, 14–21 (2018). https://doi.org/10.1016/j.ensm.2018.03.003
- F. Ming, H. Liang, Y. Lei, S. Kandambeth, M. Eddaoudi, H.N. Alshareef, Layered MgxV2O5·nH2O as cathode material for high-performance aqueous zinc ion batteries. ACS Energy Lett. 3(10), 2602–2609 (2018). https://doi.org/10.1021/acsenergylett.8b01423
References
H. Chen, M. Ling, L. Hencz, H.Y. Ling, G. Li, Z. Lin, G. Liu, S. Zhang, Exploring chemical, mechanical, and electrical functionalities of binders for advanced energy-storage devices. Chem. Rev. 118(18), 8936–8982 (2018). https://doi.org/10.1021/acs.chemrev.8b00241
Z.P. Cano, D. Banham, S. Ye, A. Hintennach, J. Lu, M. Fowler, Z. Chen, Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 3(4), 279–289 (2018). https://doi.org/10.1038/s41560-018-0108-1
J. Mei, T. Liao, L. Kou, Z. Sun, Two-dimensional metal oxide nanomaterials for next-generation rechargeable batteries. Adv. Mater. 29(48), 1700176 (2017). https://doi.org/10.1002/adma.201700176
Z.W. Chang, J.J. Xu, X.B. Zhang, Recent progress in electrocatalyst for Li-O2 batteries. Adv. Energy Mater. 7(23), 1700875 (2017). https://doi.org/10.1002/aenm.201700875
M. Winter, B. Barnett, K. Xu, Before Li ion batteries. Chem. Rev. 118(23), 11433–11456 (2018). https://doi.org/10.1021/acs.chemrev.8b00422
F.A. Susai, H. Sclar, Y. Shilina, T.R. Penki, R. Raman et al., Horizons for Li-ion batteries relevant to electro-mobility: high-specific-energy cathodes and chemically active separators. Adv. Mater. 30(41), 1801348 (2018). https://doi.org/10.1002/adma.201801348
J.-M. Tarascon, M. Armand, Issues and challenges facing rechargeable lithium batteries. Nature 414(6861), 359–367 (2001). https://doi.org/10.1038/35104644
C. Zhan, T. Wu, J. Lu, K. Amine, Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes: a critical review. Energy Environ. Sci. 11(2), 243–257 (2018). https://doi.org/10.1039/C7EE03122J
W. Zhou, S. Wang, Y. Li, S. Xin, A. Manthiram, J.B. Goodenough, Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte. J. Am. Chem. Soc. 138(30), 9385–9388 (2016). https://doi.org/10.1021/jacs.6b05341
L. Shen, L. Yu, X.Y. Yu, X. Zhang, X.W. Lou, Self-templated formation of uniform NiCo2O4 hollow spheres with complex interior structures for lithium-ion batteries and supercapacitors. Angew. Chem. Int. Ed. 54(6), 1868–1872 (2015). https://doi.org/10.1002/anie.201409776
P.K. Nayak, L. Yang, W. Brehm, P. Adelhelm, From lithium-ion to sodium-ion batteries: advantages, challenges, and surprises. Angew. Chem. Int. Ed. 57(1), 102–120 (2018). https://doi.org/10.1002/ange.201703772
Z. Zhang, S. Dong, Z. Cui, A. Du, G. Li, G. Cui, Rechargeable magnesium batteries using conversion-type cathodes: a perspective and minireview. Small Methods 2(10), 1800020 (2018). https://doi.org/10.1002/smtd.201800020
X. Yu, B. Wang, D. Gong, Z. Xu, B. Lu, Graphene nanoribbons on highly porous 3D graphene for high-capacity and ultrastable Al-ion batteries. Adv. Mater. 29(4), 1604118 (2017). https://doi.org/10.1002/adma.201604118
M. Song, H. Tan, D. Chao, H.J. Fan, Recent advances in Zn-ion batteries. Adv. Funct. Mater. 28(41), 1802564 (2018). https://doi.org/10.1002/adfm.201802564
H. Li, L. McRae, C.J. Firby, A.Y. Elezzabi, Rechargeable aqueous electrochromic batteries utilizing Ti-substituted tungsten molybdenum oxide based Zn2+ ion intercalation cathodes. Adv. Mater. 31(15), 1807065 (2019). https://doi.org/10.1002/adma.201807065
W. Xu, Y. Wang, Recent progress on zinc-ion rechargeable batteries. Nano-Micro Lett. 11, 90 (2019). https://doi.org/10.1007/s40820-019-0322-9
Y.R. Qi, Y.X. Lu, F.X. Ding, Q.Q. Zhang, H. Li, J. Huang, L.Q. Chen, Y.S. Hu, Slope-dominated carbon anode with high specific capacity and superior rate capability for high safety Na-ion batteries. Angew. Chem. Int. Ed. 58(13), 4361–4365 (2019). https://doi.org/10.1002/anie.201900005
Q. Liang, F. Chen, S. Wang, Q. Ru, Q. He, X. Hou, C.Y. Su, Y. Shi, An organic flow desalination battery. Energy Storage Mater. 20, 203–207 (2019). https://doi.org/10.1016/j.ensm.2018.11.006
H. Pan, Y. Shao, P. Yan, Y. Cheng, K.S. Han et al., Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 1, 16039 (2016). https://doi.org/10.1038/nenergy.2016.39
N. Zhang, F. Cheng, Y. Liu, Q. Zhao, K. Lei, C. Chen, X. Liu, J. Chen, Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J. Am. Chem. Soc. 138(39), 12894–12901 (2016). https://doi.org/10.1021/jacs.6b05958
N. Zhang, F. Cheng, J. Liu, L. Wang, X. Long, X. Liu, F. Li, J. Chen, Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 8, 405 (2017). https://doi.org/10.1038/s41467-017-00467-x
T. Luo, S. Wang, X. Hou, F. Chen, X. Liu, Z. Shao, H. Yan, Cr–Zn redox battery with NiFe2O4 as catalyst for enhanced degradation of Cr(VI) pollution. ACS Sustain. Chem. Eng. 7(1), 111–116 (2019). https://doi.org/10.1021/acssuschemeng.8b05299
R. Trócoli, G. Kasiri, F. La Mantia, Phase transformation of copper hexacyanoferrate (KCuFe(CN)6) during zinc insertion: effect of co-ion intercalation. J. Power Sources 400, 167–171 (2018). https://doi.org/10.1016/j.jpowsour.2018.08.015
G. Li, Z. Yang, Y. Jiang, C. Jin, W. Huang, X. Ding, Y. Huang, Towards polyvalent ion batteries: a zinc-ion battery based on NASICON structured Na3V2(PO4)3. Nano Energy 25, 211–217 (2016). https://doi.org/10.1016/j.nanoen.2016.04.051
L. Shan, J. Zhou, M. Han, G. Fang, X. Cao, X. Wu, S. Liang, Reversible Zn-driven reduction displacement reaction in aqueous zinc-ion battery. J. Mater. Chem. A 7(13), 7355–7359 (2019). https://doi.org/10.1039/C9TA00125E
X. Hou, Q. Liang, X. Hu, Y. Zhou, Q. Ru, F. Chen, S. Hu, Coupling desalination and energy storage with redox flow electrodes. Nanoscale 10(26), 12308–12314 (2018). https://doi.org/10.1039/C8NR02737D
B. Jiang, C. Xu, C. Wu, L. Dong, J. Li, F. Kang, Manganese sesquioxide as cathode material for multivalent zinc ion battery with high capacity and long cycle life. Electrochim. Acta 229, 422–428 (2017). https://doi.org/10.1016/j.electacta.2017.01.163
Y. Liu, X. Zhou, R. Liu, X. Li, Y. Bai, H. Xiao, Y. Wang, G. Yuan, Tailoring three-dimensional composite architecture for advanced zinc-ion batteries. ACS Appl. Mater. Interfaces 11(21), 19191–19199 (2019). https://doi.org/10.1021/acsami.9b04583
F. Cheng, H. Wang, Z. Zhu, Y. Wang, T. Zhang, Z. Tao, J. Chen, Porous LiMn2O4 nanorods with durable high-rate capability for rechargeable Li-ion batteries. Energy Environ. Sci. 4(9), 3668–3675 (2011). https://doi.org/10.1039/C1EE01795K
D. Wang, D. Choi, Z. Yang, V.V. Viswanathan, Z. Nie et al., Synthesis and Li-ion insertion properties of highly crystalline mesoporous rutile TiO2. Chem. Mater. 20(10), 3435–3442 (2008). https://doi.org/10.1021/cm8002589
X. Li, G. Wu, X. Liu, W. Li, M. Li, Orderly integration of porous TiO2(B) nanosheets into bunchy hierarchical structure for high-rate and ultralong-lifespan lithium-ion batteries. Nano Energy 31, 1–8 (2017). https://doi.org/10.1016/j.nanoen.2016.11.002
A.S. Poyraz, C.H. Kuo, S. Biswas, C.K. King’ondu, S.L. Suib, A general approach to crystalline and monomodal pore size mesoporous materials. Nat. Commun. 4, 2952 (2013). https://doi.org/10.1038/ncomms3952
D. Feng, T.-N. Gao, M. Fan, A. Li, K. Li, T. Wang, Q. Huo, Z.-A. Qiao, A general ligand-assisted self-assembly approach to crystalline mesoporous metal oxides. NPG Asia Mater. 10, 800–809 (2018). https://doi.org/10.1038/s41427-018-0072-z
L. Zhang, H. Dai, Y. Xia, H. Jiang, H. Zhang, H. He, Ultrasound-assisted nanocasting fabrication of ordered mesoporous MnO2 and Co3O4 with high surface areas and polycrystalline walls. J. Phys. Chem. C 114(6), 2694–2700 (2010). https://doi.org/10.1021/jp910159b
X. Zhou, Y. Zhu, W. Luo, Y. Ren, P. Xu et al., Chelation-assisted soft-template synthesis of ordered mesoporous zinc oxides for low concentration gas sensing. J. Mater. Chem. A 4(39), 15064–15071 (2016). https://doi.org/10.1039/c6ta05687c
P. Kar, S. Sardar, S. Ghosh, M.R. Parida, B. Liu, O.F. Mohammed, P. Lemmens, S.K. Pal, Nano surface engineering of Mn2O3 for potential light-harvesting application. J. Mater. Chem. C 3(31), 8200–8211 (2015). https://doi.org/10.1039/C5TC01475A
M.S. Kolathodi, S.N. Hanumantha Rao, T.S. Natarajan, G. Singh, Beaded manganese oxide (Mn2O3) nanofibers: preparation and application for capacitive energy storage. J. Mater. Chem. A 4(20), 7883–7891 (2016). https://doi.org/10.1039/C6TA01948J
D. Ji, H. Zhou, J. Zhang, Y. Dan, H. Yang, A. Yuan, Facile synthesis of a metal–organic framework-derived Mn2O3 nanowire coated three-dimensional graphene network for high-performance freestanding supercapacitor electrodes. J. Mater. Chem. A 4(21), 8283–8290 (2016). https://doi.org/10.1039/c6ta01377e
K. Möller, J. Kobler, T. Bein, Colloidal suspensions of nanometer-sized mesoporous silica. Adv. Funct. Mater. 17(4), 605–612 (2007). https://doi.org/10.1002/adfm.200600578
S.D. Han, S. Kim, D. Li, V. Petkov, H.D. Yoo et al., Mechanism of Zn insertion into nanostructured δ-MnO2: a nonaqueous rechargeable Zn metal battery. Chem. Mater. 29(11), 4874–4884 (2017). https://doi.org/10.1021/acs.chemmater.7b00852
S. Islam, M.H. Alfaruqi, V. Mathew, J. Song, S. Kim et al., Facile synthesis and the exploration of the zinc storage mechanism of β-MnO2 nanorods with exposed (101) planes as a novel cathode material for high performance eco-friendly zinc-ion batteries. J. Mater. Chem. A 5(44), 23299–23309 (2017). https://doi.org/10.1039/c7ta07170a
W. Sun, F. Wang, S. Hou, C. Yang, X. Fan et al., Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J. Am. Chem. Soc. 139(29), 9775–9778 (2017). https://doi.org/10.1021/jacs.7b04471
B. Tang, G. Fang, J. Zhou, L. Wang, Y. Lei et al., Potassium vanadates with stable structure and fast ion diffusion channel as cathode for rechargeable aqueous zinc-ion batteries. Nano Energy 51, 579–587 (2018). https://doi.org/10.1016/j.nanoen.2018.07.014
L. Ma, S. Chen, H. Li, Z. Ruan, Z. Tang et al., Initiating a mild aqueous electrolyte Co3O4/Zn battery with 2.2 V-high voltage and 5000-cycle lifespan by a Co(III) rich-electrode. Energy Environ. Sci. 11(9), 2521–2530 (2018). https://doi.org/10.1039/C8EE01415A
Z. Guo, Y. Ma, X. Dong, J. Huang, Y. Wang, Y. Xia, An environmentally friendly and flexible aqueous zinc battery using an organic cathode. Angew. Chem. Int. Ed. 57(36), 11737–11741 (2018). https://doi.org/10.1002/anie.201807121
C. Pan, R.G. Nuzzo, A.A. Gewirth, ZnAlxCo2−xO4 spinels as cathode materials for non-aqueous Zn batteries with an open circuit voltage of ≤ 2 V. Chem. Mater. 29(21), 9351–9359 (2017). https://doi.org/10.1021/acs.chemmater.7b03340
B. Sambandam, V. Soundharrajan, S. Kim, M.H. Alfaruqi, J. Jo et al., Aqueous rechargeable Zn-ion batteries: an imperishable and high-energy Zn2V2O7 nanowire cathode through intercalation regulation. J. Mater. Chem. A 6(9), 3850–3856 (2018). https://doi.org/10.1039/c7ta11237h
W. Li, K. Wang, S. Cheng, K. Jiang, A long-life aqueous Zn-ion battery based on Na3V2(PO4)2F3 cathode. Energy Storage Mater. 15, 14–21 (2018). https://doi.org/10.1016/j.ensm.2018.03.003
F. Ming, H. Liang, Y. Lei, S. Kandambeth, M. Eddaoudi, H.N. Alshareef, Layered MgxV2O5·nH2O as cathode material for high-performance aqueous zinc ion batteries. ACS Energy Lett. 3(10), 2602–2609 (2018). https://doi.org/10.1021/acsenergylett.8b01423