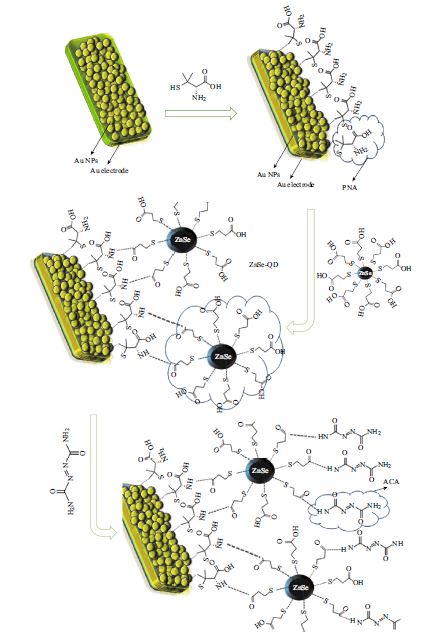
The Electrochemical Behavior of Au/AuNPs/PNA/ZnSe-QD/ACA Electrode Towards CySH Oxidation
Corresponding Author: Azadeh Azadbakht
Nano-Micro Letters,
Vol. 7 No. 2 (2015), Article Number: 152-164
Abstract
This work describes the electrochemical behavior of azodicarboxamide (ACA) film immobilized on the surface of penicillamine (PNA)/ZnSe-quantum dot (ZnSe-QD) gold nanoparticle (AuNPs) Au electrode. Electrocatalytic activity of modified electrode toward the oxidation of cysteine (CySH) was investigated. The surface structure and composition of the sensor were characterized by scanning electron microscopy (SEM). Oxidation of CySH on the surface of modified electrode was investigated with cyclic voltammetry, electrochemical impedance spectroscopy (EIS), hydrodynamic voltammetry and chronoamperometry methods. The results show that the PNA/ZnSe-QD/ACA film displays excellent electrochemical catalytic activities towards CySH oxidation. The modified electrode shows reproducible behavior and high level of stability during the electrochemical experiments. Also it has short response time, low detection limit, high sensitivity and low operation potential, which can be used as an amperometric sensor for monitoring of CySH. The proposed modified electrode was successfully used for determination of CySH in real sample such as human serum.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- J.C. Claussen, A.D. Franklin, A. Haque, D.M. Porterfield, T.S. Fisher, Electrochemical biosensor of nanocube-augmented carbon nanotube networks. ACS Nano 3(1), 37–44 (2009). doi:10.1021/nn800682m
- T.G. Drummond, M.G. Hill, J.K. Barton, Electrochemical DNA sensors. Nat. Biotechnol. 21(10), 1192–1199 (2003). doi:10.1038/nbt873
- F. Patolsky, G. Zheng, C.M. Lieber, Nanowire sensors for medicine and the life sciences. Nanomedicine 1(1), 51–65 (2006). doi:10.2217/17435889.1.1.51
- M. Hung, D.M. Stanbury, Catalytic and direct oxidation of cysteine by octacyanomolybdate(V). Inorg. Chem. 44(10), 3541–3550 (2005). doi:10.1021/ic050427c
- T. Inoue, J.R. Kirchhoff, Electrochemical detection of thiols with a coenzyme pyrroloquinoline quinone modified electrode. Anal. Chem. 72(23), 5755–5760 (2000). doi:10.1021/ac000716c
- M.M. Ardakani, P. Rahimi, P.E. Karami, H.R. Zare, H. Naeimi, Electrocatalytic oxidation of cysteine by quinizarine at glassy carbon electrode. Sens. Actuators B 123(2), 763–768 (2007). doi:10.1016/j.snb.2006.10.015
- S. Shahrokhian, Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode. Anal. Chem. 73(24), 5972–5978 (2001). doi:10.1021/ac010541m
- P.C. White, N.S. Lawrence, J. Davis, R.G. Compton, Electrochemically initiated 1, 4 additions: a versatile route to the determination of thiols. Anal. Chim. Acta 447(1–2), 1–10 (2001). doi:10.1016/S0003-2670(01)01297-1
- S. Seshadri, A. Beiser, J. Selhub, P.F. Jacques, I.H. Rosenberg, R.B. D’Agostino, P.W.F.N. Wilson, P.A. Wolf, Plasma homocysteine as a risk factor for dementia and alzheimer’s disease. N. Engl. J. Med. 346(7), 476–483 (2002). doi:10.1056/NEJMoa011613
- M.A. Hofmann, B. Kohl, M.S. Zumbach, V. Borcea, A. Bierhaus, M. Henkels, J. Amiral, W. Fiehn, R. Ziegler, P. Wahl, P.P. Nawroth, Hyperhomocyst(e)inemia and endothelial dysfunction in IDDM. Diabetes Care 20(12), 1880–1886 (1997). doi:10.2337/diacare.20.12.1880
- E.K. Hoogeveen, P.J. Kostense, P.J. Beks, A.J.C. Mackaay, C. Jakobs, L.M. Bouter, R.J. Heine, C.D. Stehouwer, Hyper homocysteinemia is associated with an increased risk of cardiovascular disease, especially in non–insulin dependent diabetes mellitus: a population-based study. Arterioscler. Thromb. Vasc. Biol. 18(1), 133–138 (1998). doi:10.1161/01.ATV.18.1.133
- B. Hultberg, E. Agardh, A. Andersson, L. Brattstrom, A. Isaksson, B. Israelsson, C.D. Agardh, Increased levels of plasma homocysteine are associated with nephropathy, but not severe retinopathy in type 1 diabetes mellitus. Scand. J. Clin. Lab. Inv. 51(3), 277–282 (1991). doi:10.3109/00365519109091615
- E. Sharifi, A. Salimi, E. Shams, DNA/nickel oxide nanoparticles/osmium(III)-complex modified electrode toward selective oxidation of l-cysteine and simultaneous detection of l-cysteine and homocysteine. Bioelectrochemistry 86(6), 9–21 (2012). doi:10.1016/j.bioelechem.2011.12.013
- W. Wang, O. Rusin, X. Xu, K.K. Kim, J.O. Escobedo, S.O. Fakayode, K.A. Fletcher, M. Lowry, C.M. Schowalter, C.M. Lawrence, F.R. Fronczek, I.M. Warner, R.M. Strongin, Detection of homocysteine and cysteine. J. Am. Chem. Soc. 127(45), 15949–15958 (2005)
- G. Chwatko, E. Bald, Determination of cysteine in human plasma by high-performance liquid chromatography and ultraviolet detection after pre-column derivatization with 2-chloro-1-methylpyridinium iodide. Talanta 52(3), 509–515 (2000). doi:10.1016/S0039-9140(00)00394-5
- A. Sano, H. Nakamura, Chemiluminescence detection of thiols by high-performance liquid chromatography using o-Phthalaldehyde and N-(4-Aminobutyl)-N-ethylisoluminol as precolumn derivatization reagents. Anal. Sci. 14(4), 731–737 (1998). doi:10.2116/analsci.14.731
- K. Arlt, S. Brandt, J. Kehr, Amino acid analysis in five pooled single plant cell samples using capillary electrophoresis coupled to laser-induced fluorescence detection. J. Chromatogr. A 926(2), 319–325 (2001). doi:10.1016/S0021-9673(01)01052-4
- M. Ummadi, B.C. Weimer, Use of capillary electrophoresis and laser-induced fluorescence for attomole detection of amino acids. J. Chromatogr. A 964(1–2), 243–253 (2002). doi:10.1016/S0021-9673(02)00692-1
- F. Tanaka, N. Mase, C.F. Barbas, Determination of cysteine concentration by fluorescence increase: reaction of cysteine with a fluorogenic aldehyde. Chem. Commun. 5, 1762–1763 (2004). doi:10.1039/b405642f
- D.A.M. Zaia, K.C.L. Ribas, C.T.B.V. Zaia, Spectrophotometric determination of cysteine and/or carbocysteine in a mixture of amino acids, shampoo, and pharmaceutical products using p-benzoquinone. Talanta 50(5), 1003–1010 (1999). doi:10.1016/S0039-9140(99)00218-0
- G. Shi, J. Lu, F. Xu, W. Sun, L. Jin, K. Yamamoto, S. Tao, J. Jin, Determination of glutathione in vivo by microdialysis using liquid chromatography with a cobalt hexacyanoferrate chemically modified electrode. Anal. Chim. Acta 391(4), 307–313 (1999). doi:10.1016/S0003-2670(99)00274-3
- F. Pak, K. Meral, R. Altundaş, D. Ekinci, Self-assembled monolayers of fluorene- and nitrofluorene-terminated thiols on polycrystalline gold electrode: electrochemical and optical properties. J. Electroanal. Chem. 654(1–2), 20–28 (2011). doi:10.1016/j.jelechem.2011.01.041
- S.M. Chen, J.Y. Chen, R. Thangamuthu, Electrochemical preparation of brilliant-blue-modified poly(diallyldimethylammoniumchloride) and nafion-coated glassy carbon electrodes and their electrocatalytic behavior towards oxygen and l-cysteine. Electroanalysis 20(14), 1565–1573 (2008). doi:10.1002/elan.200804213
- S. Ge, M. Yan, J. Lu, M. Zhang, F. Yu, J. Yu, X. Song, S. Yu, Electrochemical biosensor based on graphene oxide–Au nanoclusters composites for l-cysteine analysis. Biosen. Bioelectron. 31(1), 49–54 (2012). doi:10.1016/j.bios.2011.09.038
- H. Hosseini, H. Ahmar, A. Dehghani, A. Bagheri, A. Tadjarodi, A.R. Fakhari, A novel electrochemical sensor based on metal-organic framework for electro-catalytic oxidation of l-cysteine. Biosen. Bioelectron. 42, 426–429 (2013). doi:10.1016/j.bios.2012.09.062
- M. Zhou, J. Ding, L.-P. Guo, Q.-K. Shang, Electrochemical behavior of l-cysteine and its detection at ordered mesoporouscarbon-modified glassy carbon electrode. Anal. Chem. 79(14), 5328–5335 (2007). doi:10.1021/ac0703707
- M. Liu, G. Shi, L. Zhang, Y. Cheng, L. Jin, Quantum dots modified electrode and its application in electroanalysis of hemoglobin. Electrochem. Commun. 8(2), 305–310 (2006). doi:10.1016/j.elecom.2005.11.026
- J. Drbohlavova, V. Adam, R. Kizek, J. Hubalek, Quantum dots-characterization, preparation and usage in biological systems. Int. J. Mol. Sci. 10(2), 656–673 (2009). doi:10.3390/ijms10020656
- J. Aldana, Y.A. Wang, X. Peng, Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. J. Am. Chem. Soc. 123(36), 8844–8850 (2001). doi:10.1021/ja016424q
- M.J. Giz, B. Duong, N.J. Tao, In situ STM study of self-assembled mercaptopropionic acid monolayers for electrochemical detection of dopamine. J. Electroanal. Chem. 465(1), 72–79 (1999). doi:10.1016/S0022-0728(99)00056-X
- J. Li, G. Zou, X. Hu, X. Zhang, Electrochemistry of thiol-capped CdTe quantum dots and its sensing application. J. Electroanal. Chem. 625(1), 88–91 (2009). doi:10.1016/j.jelechem.2008.10.011
- J. Berna, M. Alajarín, R.A. Orenes, Azodicarboxamides as template binding motifs for the building of hydrogen-bonded molecular shuttles. J. Am. Chem. Soc. 132(31), 10741–10747 (2010). doi:10.1021/ja101151t
- H. Cui, Y. Xu, Z.F. Zhang, Multichannel electrochemiluminescence of luminol in neutral and alkaline aqueous solutions on a gold nanoparticle self-assembled electrode. Anal. Chem. 76(14), 4002–4010 (2004). doi:10.1021/ac049889i
- J.J. Andrade, J.A. Brasil Jr, P.M.A. Farias, A. Fontes, B.S. Santos, Synthesis and characterization of blue emitting ZnSe quantum dots. Microelectron. J. 40(3), 641–643 (2009). doi:10.1016/j.mejo.2008.06.040
- V. Swayambunathan, D. Hayes, K.H. Schmidt, Y.X. Liao, D. Meisel, Thiol surface complexation on growing cadmium sulfide clusters. J. Am. Chem. Soc. 112(10), 3831–3837 (1990). doi:10.1021/ja00166a017
- M.B. Gholivand, A. Azadbakht, Fabrication of a highly sensitive glucose electrochemical sensor based on immobilization of Ni(II)–pyromellitic acid and bimetallic Au–Pt inorganic–organic hybrid nanocomposite onto carbon nanotube modified glassy carbon electrode. Electrochim. Acta 76, 300–311 (2012). doi:10.1016/j.electacta.2012.05.037
- S. Antoniadou, A.D. Jannakoudakis, E. Theodoridou, Electrocatalytic reactions on carbon fibre electrodes modified by hemine II. Electro-oxidation of hydrazine. Synth. Met. 30(3), 295–304 (1980). doi:10.1016/0379-6779(89)90652-8
- E. Laviron, General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 101(1), 19–28 (1979). doi:10.1016/S0022-0728(79)80075-3
- A.J. Bard, L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications, 2nd edn. (Wiley, New York, 2000)
- J.A. Harrison, Z.A. Khan, The oxidation of hydrazine on platinum in acid solution. J. Electroanal. Chem. 28(1), 131–138 (1970). doi:10.1016/S0022-0728(70)80288-1
- T.R. Ralph, M.L. Hitchman, J.P. Millington, F.C. Walsh, The electrochemistry of l-cystine and l-cysteine: part 1: Thermodynamic and kinetic studies. J. Electroanal. Chem. 375(1–2), 1–15 (1994). doi:10.1016/0022-0728(94)03407-9
- Z. Galus, Fundamentals of Electrochemical Analysis (Horwood, New York, 1976)
- X. Tang, Y. Liu, H. Hou, T. You, Electrochemical determination of l-Tryptophan, l-Tyrosine and l-Cysteine using electrospun carbon nanofibers modified electrode. Talanta 80(5), 2182–2186 (2010). doi:10.1016/j.talanta.2009.11.027
- R. Ojani, J.B. Raoof, E. Zarei, Preparation of poly N, N-dimethylaniline/ferrocyanide film modified carbon paste electrode: application to electrocatalytic oxidation of l-cysteine. J. Electroanal. Chem. 638(2), 241–245 (2010). doi:10.1016/j.jelechem.2009.11.005
- J.C. Ndamanisha, J. Bai, B. Qi, L. Guo, Application of electrochemical properties of ordered mesoporous carbon to the determination of glutathione and cysteine. Anal. Biochem. 386(1), 79–84 (2009). doi:10.1016/j.ab.2008.11.041
- J.M. Zen, A.S. Kumar, J.-C. Chen, Electrocatalytic oxidation and sensitive detection of cysteine on a lead ruthenate pyrochlore modified electrode. Anal. Chem. 73(6), 1169–1175 (2001). doi:10.1021/ac0010781
- M.K. Amini, J.H. Khorasani, S.S. Khaloo, S. Tangestaninejad, Cobalt(II) salophen-modified carbon-paste electrode for potentiometric and voltammetric determination of cysteine. Anal. Biochem. 320(1), 32–38 (2003). doi:10.1016/S0003-2697(03)00355-5
- A. Salimi, S. Pourbeyram, Renewable sol–gel carbon ceramic electrodes modified with a Ru-complex for the amperometric detection of l-cysteine and glutathione. Talanta 60(1), 205–214 (2003). doi:10.1016/S0039-9140(03)00125-5
- A. Salimi, R. Hallaj, Catalytic oxidation of thiols at preheated glassy carbon electrode modified with abrasive immobilization of multiwall carbon nanotubes: applications to amperometric detection of thiocytosine, l-cysteine and glutathione. Talanta 66(4), 967–975 (2005). doi:10.1016/j.talanta.2004.12.040
- W.Y. Su, S.H. Cheng, Electrocatalysis and sensitive determination of cysteine at poly(3,4-ethylenedioxythiophene)-modified screen-printed electrodes. Electrochem. Commun. 10(6), 899–902 (2008). doi:10.1016/j.elecom.2008.04.013
- A. Abbaspour, A. Ghaffarinejad, Electrocatalytic oxidation of l-cysteine with a stable copper–cobalt hexacyanoferrate electrochemically modified carbon paste electrode. Electrochim. Acta 53(22), 6643–6650 (2008). doi:10.1016/j.electacta.2008.04.065
- Y.P. Dong, L. Pei, X.F. Chu, W.B. Zhang, Q.F. Zhang, Electrochemical behavior of cysteine at a CuGeO3 nanowires modified glassy carbon electrode. Electrochim. Acta 55(18), 5135–5141 (2010). doi:10.1016/j.electacta.2010.04.020
- P. Sweth, A.S. Kumar, Phosphomolybdic acid nano-aggregates immobilized nafion membrane modified electrode for selective cysteine electrocatalytic oxidation and anti-dermatophytic activity. Electrochim. Acta 98, 54–65 (2013). doi:10.1016/j.electacta.2013.03.023
- H. Razmi, A. Azadbakht, Electrochemical characteristics of dopamine oxidation at palladium hexacyanoferrate film, electroless plated on aluminum electrode. Electrochim. Acta 50(11), 2193–2201 (2005). doi:10.1016/j.electacta.2004.10.001
- A.A. Ensafi, S. Behyan, Sensing of l-cysteine at glassy carbon electrode using nile blue A as a mediator. Sens. Actuators B 122(1), 282–288 (2007). doi:10.1016/j.snb.2006.05.035
- S.P. Stabler, P.D. Marcell, E.R. Podell, R.H. Allen, Quantitation of total homocysteine, total cysteine, and methionine in normal serum and urine using capillary gas chromatography-mass spectrometry. Anal. Biochem. 162(1), 185–196 (1987). doi:10.1016/0003-2697(87)90026-1
References
J.C. Claussen, A.D. Franklin, A. Haque, D.M. Porterfield, T.S. Fisher, Electrochemical biosensor of nanocube-augmented carbon nanotube networks. ACS Nano 3(1), 37–44 (2009). doi:10.1021/nn800682m
T.G. Drummond, M.G. Hill, J.K. Barton, Electrochemical DNA sensors. Nat. Biotechnol. 21(10), 1192–1199 (2003). doi:10.1038/nbt873
F. Patolsky, G. Zheng, C.M. Lieber, Nanowire sensors for medicine and the life sciences. Nanomedicine 1(1), 51–65 (2006). doi:10.2217/17435889.1.1.51
M. Hung, D.M. Stanbury, Catalytic and direct oxidation of cysteine by octacyanomolybdate(V). Inorg. Chem. 44(10), 3541–3550 (2005). doi:10.1021/ic050427c
T. Inoue, J.R. Kirchhoff, Electrochemical detection of thiols with a coenzyme pyrroloquinoline quinone modified electrode. Anal. Chem. 72(23), 5755–5760 (2000). doi:10.1021/ac000716c
M.M. Ardakani, P. Rahimi, P.E. Karami, H.R. Zare, H. Naeimi, Electrocatalytic oxidation of cysteine by quinizarine at glassy carbon electrode. Sens. Actuators B 123(2), 763–768 (2007). doi:10.1016/j.snb.2006.10.015
S. Shahrokhian, Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode. Anal. Chem. 73(24), 5972–5978 (2001). doi:10.1021/ac010541m
P.C. White, N.S. Lawrence, J. Davis, R.G. Compton, Electrochemically initiated 1, 4 additions: a versatile route to the determination of thiols. Anal. Chim. Acta 447(1–2), 1–10 (2001). doi:10.1016/S0003-2670(01)01297-1
S. Seshadri, A. Beiser, J. Selhub, P.F. Jacques, I.H. Rosenberg, R.B. D’Agostino, P.W.F.N. Wilson, P.A. Wolf, Plasma homocysteine as a risk factor for dementia and alzheimer’s disease. N. Engl. J. Med. 346(7), 476–483 (2002). doi:10.1056/NEJMoa011613
M.A. Hofmann, B. Kohl, M.S. Zumbach, V. Borcea, A. Bierhaus, M. Henkels, J. Amiral, W. Fiehn, R. Ziegler, P. Wahl, P.P. Nawroth, Hyperhomocyst(e)inemia and endothelial dysfunction in IDDM. Diabetes Care 20(12), 1880–1886 (1997). doi:10.2337/diacare.20.12.1880
E.K. Hoogeveen, P.J. Kostense, P.J. Beks, A.J.C. Mackaay, C. Jakobs, L.M. Bouter, R.J. Heine, C.D. Stehouwer, Hyper homocysteinemia is associated with an increased risk of cardiovascular disease, especially in non–insulin dependent diabetes mellitus: a population-based study. Arterioscler. Thromb. Vasc. Biol. 18(1), 133–138 (1998). doi:10.1161/01.ATV.18.1.133
B. Hultberg, E. Agardh, A. Andersson, L. Brattstrom, A. Isaksson, B. Israelsson, C.D. Agardh, Increased levels of plasma homocysteine are associated with nephropathy, but not severe retinopathy in type 1 diabetes mellitus. Scand. J. Clin. Lab. Inv. 51(3), 277–282 (1991). doi:10.3109/00365519109091615
E. Sharifi, A. Salimi, E. Shams, DNA/nickel oxide nanoparticles/osmium(III)-complex modified electrode toward selective oxidation of l-cysteine and simultaneous detection of l-cysteine and homocysteine. Bioelectrochemistry 86(6), 9–21 (2012). doi:10.1016/j.bioelechem.2011.12.013
W. Wang, O. Rusin, X. Xu, K.K. Kim, J.O. Escobedo, S.O. Fakayode, K.A. Fletcher, M. Lowry, C.M. Schowalter, C.M. Lawrence, F.R. Fronczek, I.M. Warner, R.M. Strongin, Detection of homocysteine and cysteine. J. Am. Chem. Soc. 127(45), 15949–15958 (2005)
G. Chwatko, E. Bald, Determination of cysteine in human plasma by high-performance liquid chromatography and ultraviolet detection after pre-column derivatization with 2-chloro-1-methylpyridinium iodide. Talanta 52(3), 509–515 (2000). doi:10.1016/S0039-9140(00)00394-5
A. Sano, H. Nakamura, Chemiluminescence detection of thiols by high-performance liquid chromatography using o-Phthalaldehyde and N-(4-Aminobutyl)-N-ethylisoluminol as precolumn derivatization reagents. Anal. Sci. 14(4), 731–737 (1998). doi:10.2116/analsci.14.731
K. Arlt, S. Brandt, J. Kehr, Amino acid analysis in five pooled single plant cell samples using capillary electrophoresis coupled to laser-induced fluorescence detection. J. Chromatogr. A 926(2), 319–325 (2001). doi:10.1016/S0021-9673(01)01052-4
M. Ummadi, B.C. Weimer, Use of capillary electrophoresis and laser-induced fluorescence for attomole detection of amino acids. J. Chromatogr. A 964(1–2), 243–253 (2002). doi:10.1016/S0021-9673(02)00692-1
F. Tanaka, N. Mase, C.F. Barbas, Determination of cysteine concentration by fluorescence increase: reaction of cysteine with a fluorogenic aldehyde. Chem. Commun. 5, 1762–1763 (2004). doi:10.1039/b405642f
D.A.M. Zaia, K.C.L. Ribas, C.T.B.V. Zaia, Spectrophotometric determination of cysteine and/or carbocysteine in a mixture of amino acids, shampoo, and pharmaceutical products using p-benzoquinone. Talanta 50(5), 1003–1010 (1999). doi:10.1016/S0039-9140(99)00218-0
G. Shi, J. Lu, F. Xu, W. Sun, L. Jin, K. Yamamoto, S. Tao, J. Jin, Determination of glutathione in vivo by microdialysis using liquid chromatography with a cobalt hexacyanoferrate chemically modified electrode. Anal. Chim. Acta 391(4), 307–313 (1999). doi:10.1016/S0003-2670(99)00274-3
F. Pak, K. Meral, R. Altundaş, D. Ekinci, Self-assembled monolayers of fluorene- and nitrofluorene-terminated thiols on polycrystalline gold electrode: electrochemical and optical properties. J. Electroanal. Chem. 654(1–2), 20–28 (2011). doi:10.1016/j.jelechem.2011.01.041
S.M. Chen, J.Y. Chen, R. Thangamuthu, Electrochemical preparation of brilliant-blue-modified poly(diallyldimethylammoniumchloride) and nafion-coated glassy carbon electrodes and their electrocatalytic behavior towards oxygen and l-cysteine. Electroanalysis 20(14), 1565–1573 (2008). doi:10.1002/elan.200804213
S. Ge, M. Yan, J. Lu, M. Zhang, F. Yu, J. Yu, X. Song, S. Yu, Electrochemical biosensor based on graphene oxide–Au nanoclusters composites for l-cysteine analysis. Biosen. Bioelectron. 31(1), 49–54 (2012). doi:10.1016/j.bios.2011.09.038
H. Hosseini, H. Ahmar, A. Dehghani, A. Bagheri, A. Tadjarodi, A.R. Fakhari, A novel electrochemical sensor based on metal-organic framework for electro-catalytic oxidation of l-cysteine. Biosen. Bioelectron. 42, 426–429 (2013). doi:10.1016/j.bios.2012.09.062
M. Zhou, J. Ding, L.-P. Guo, Q.-K. Shang, Electrochemical behavior of l-cysteine and its detection at ordered mesoporouscarbon-modified glassy carbon electrode. Anal. Chem. 79(14), 5328–5335 (2007). doi:10.1021/ac0703707
M. Liu, G. Shi, L. Zhang, Y. Cheng, L. Jin, Quantum dots modified electrode and its application in electroanalysis of hemoglobin. Electrochem. Commun. 8(2), 305–310 (2006). doi:10.1016/j.elecom.2005.11.026
J. Drbohlavova, V. Adam, R. Kizek, J. Hubalek, Quantum dots-characterization, preparation and usage in biological systems. Int. J. Mol. Sci. 10(2), 656–673 (2009). doi:10.3390/ijms10020656
J. Aldana, Y.A. Wang, X. Peng, Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. J. Am. Chem. Soc. 123(36), 8844–8850 (2001). doi:10.1021/ja016424q
M.J. Giz, B. Duong, N.J. Tao, In situ STM study of self-assembled mercaptopropionic acid monolayers for electrochemical detection of dopamine. J. Electroanal. Chem. 465(1), 72–79 (1999). doi:10.1016/S0022-0728(99)00056-X
J. Li, G. Zou, X. Hu, X. Zhang, Electrochemistry of thiol-capped CdTe quantum dots and its sensing application. J. Electroanal. Chem. 625(1), 88–91 (2009). doi:10.1016/j.jelechem.2008.10.011
J. Berna, M. Alajarín, R.A. Orenes, Azodicarboxamides as template binding motifs for the building of hydrogen-bonded molecular shuttles. J. Am. Chem. Soc. 132(31), 10741–10747 (2010). doi:10.1021/ja101151t
H. Cui, Y. Xu, Z.F. Zhang, Multichannel electrochemiluminescence of luminol in neutral and alkaline aqueous solutions on a gold nanoparticle self-assembled electrode. Anal. Chem. 76(14), 4002–4010 (2004). doi:10.1021/ac049889i
J.J. Andrade, J.A. Brasil Jr, P.M.A. Farias, A. Fontes, B.S. Santos, Synthesis and characterization of blue emitting ZnSe quantum dots. Microelectron. J. 40(3), 641–643 (2009). doi:10.1016/j.mejo.2008.06.040
V. Swayambunathan, D. Hayes, K.H. Schmidt, Y.X. Liao, D. Meisel, Thiol surface complexation on growing cadmium sulfide clusters. J. Am. Chem. Soc. 112(10), 3831–3837 (1990). doi:10.1021/ja00166a017
M.B. Gholivand, A. Azadbakht, Fabrication of a highly sensitive glucose electrochemical sensor based on immobilization of Ni(II)–pyromellitic acid and bimetallic Au–Pt inorganic–organic hybrid nanocomposite onto carbon nanotube modified glassy carbon electrode. Electrochim. Acta 76, 300–311 (2012). doi:10.1016/j.electacta.2012.05.037
S. Antoniadou, A.D. Jannakoudakis, E. Theodoridou, Electrocatalytic reactions on carbon fibre electrodes modified by hemine II. Electro-oxidation of hydrazine. Synth. Met. 30(3), 295–304 (1980). doi:10.1016/0379-6779(89)90652-8
E. Laviron, General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 101(1), 19–28 (1979). doi:10.1016/S0022-0728(79)80075-3
A.J. Bard, L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications, 2nd edn. (Wiley, New York, 2000)
J.A. Harrison, Z.A. Khan, The oxidation of hydrazine on platinum in acid solution. J. Electroanal. Chem. 28(1), 131–138 (1970). doi:10.1016/S0022-0728(70)80288-1
T.R. Ralph, M.L. Hitchman, J.P. Millington, F.C. Walsh, The electrochemistry of l-cystine and l-cysteine: part 1: Thermodynamic and kinetic studies. J. Electroanal. Chem. 375(1–2), 1–15 (1994). doi:10.1016/0022-0728(94)03407-9
Z. Galus, Fundamentals of Electrochemical Analysis (Horwood, New York, 1976)
X. Tang, Y. Liu, H. Hou, T. You, Electrochemical determination of l-Tryptophan, l-Tyrosine and l-Cysteine using electrospun carbon nanofibers modified electrode. Talanta 80(5), 2182–2186 (2010). doi:10.1016/j.talanta.2009.11.027
R. Ojani, J.B. Raoof, E. Zarei, Preparation of poly N, N-dimethylaniline/ferrocyanide film modified carbon paste electrode: application to electrocatalytic oxidation of l-cysteine. J. Electroanal. Chem. 638(2), 241–245 (2010). doi:10.1016/j.jelechem.2009.11.005
J.C. Ndamanisha, J. Bai, B. Qi, L. Guo, Application of electrochemical properties of ordered mesoporous carbon to the determination of glutathione and cysteine. Anal. Biochem. 386(1), 79–84 (2009). doi:10.1016/j.ab.2008.11.041
J.M. Zen, A.S. Kumar, J.-C. Chen, Electrocatalytic oxidation and sensitive detection of cysteine on a lead ruthenate pyrochlore modified electrode. Anal. Chem. 73(6), 1169–1175 (2001). doi:10.1021/ac0010781
M.K. Amini, J.H. Khorasani, S.S. Khaloo, S. Tangestaninejad, Cobalt(II) salophen-modified carbon-paste electrode for potentiometric and voltammetric determination of cysteine. Anal. Biochem. 320(1), 32–38 (2003). doi:10.1016/S0003-2697(03)00355-5
A. Salimi, S. Pourbeyram, Renewable sol–gel carbon ceramic electrodes modified with a Ru-complex for the amperometric detection of l-cysteine and glutathione. Talanta 60(1), 205–214 (2003). doi:10.1016/S0039-9140(03)00125-5
A. Salimi, R. Hallaj, Catalytic oxidation of thiols at preheated glassy carbon electrode modified with abrasive immobilization of multiwall carbon nanotubes: applications to amperometric detection of thiocytosine, l-cysteine and glutathione. Talanta 66(4), 967–975 (2005). doi:10.1016/j.talanta.2004.12.040
W.Y. Su, S.H. Cheng, Electrocatalysis and sensitive determination of cysteine at poly(3,4-ethylenedioxythiophene)-modified screen-printed electrodes. Electrochem. Commun. 10(6), 899–902 (2008). doi:10.1016/j.elecom.2008.04.013
A. Abbaspour, A. Ghaffarinejad, Electrocatalytic oxidation of l-cysteine with a stable copper–cobalt hexacyanoferrate electrochemically modified carbon paste electrode. Electrochim. Acta 53(22), 6643–6650 (2008). doi:10.1016/j.electacta.2008.04.065
Y.P. Dong, L. Pei, X.F. Chu, W.B. Zhang, Q.F. Zhang, Electrochemical behavior of cysteine at a CuGeO3 nanowires modified glassy carbon electrode. Electrochim. Acta 55(18), 5135–5141 (2010). doi:10.1016/j.electacta.2010.04.020
P. Sweth, A.S. Kumar, Phosphomolybdic acid nano-aggregates immobilized nafion membrane modified electrode for selective cysteine electrocatalytic oxidation and anti-dermatophytic activity. Electrochim. Acta 98, 54–65 (2013). doi:10.1016/j.electacta.2013.03.023
H. Razmi, A. Azadbakht, Electrochemical characteristics of dopamine oxidation at palladium hexacyanoferrate film, electroless plated on aluminum electrode. Electrochim. Acta 50(11), 2193–2201 (2005). doi:10.1016/j.electacta.2004.10.001
A.A. Ensafi, S. Behyan, Sensing of l-cysteine at glassy carbon electrode using nile blue A as a mediator. Sens. Actuators B 122(1), 282–288 (2007). doi:10.1016/j.snb.2006.05.035
S.P. Stabler, P.D. Marcell, E.R. Podell, R.H. Allen, Quantitation of total homocysteine, total cysteine, and methionine in normal serum and urine using capillary gas chromatography-mass spectrometry. Anal. Biochem. 162(1), 185–196 (1987). doi:10.1016/0003-2697(87)90026-1