Hydrogel Microneedle Arrays for Transdermal Drug Delivery
Corresponding Author: Weien Yuan
Nano-Micro Letters,
Vol. 6 No. 3 (2014), Article Number: 191-199
Abstract
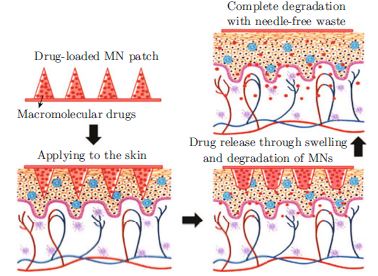
Stratum corneum is the main obstacle for drugs to pass through the skin. Microneedles are composed of arrays of micro-projections formed with different materials, generally ranging from 25–2000 μm in height. Microneedles straightly pierce the skin with its short needle arrays to overcome this barrier. Microneedles can be divided into several categories, for instance, solid microneedles, coated microneedles, and hollow microneedles and so on. However, all these types have their weak points related to corresponding mechanisms. In recent years, pioneering scientists have been working on these issues and some possible solutions have been investigated. This article will focus on the microneedle arrays consisting of hydrogels. Hydrogels are commonly used in drug delivery field. Hydrogel microneedles can be further divided into dissolving and degradable microneedles and phase transition microneedles. The former leaves drug with matrix in the skin. The latter has the feature that drugs in the matrix are delivered while the remaining ingredients can be easily removed from the skin after usage. For drugs which are required to be used every day, the phase transition microneedles are more acceptable. This article is written in order to summarize the advantages of these designs and summarize issues to be solved which may hinder the development of this technology.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- M. R. Prausnitz and R. Langer, “Transdermal drug delivery”, Nat. Biotechnol. 26(11), 1261–1268 (2008). http://dx.doi.org/10.1038/nbt.1504
- R. F. Donnelly, T. R. R. Singh and A. D. Woolfson, “Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety”, Drug Deliv. 17(4), 187–207 (2010). http://dx.doi.org/10.3109/10717541003667798
- Y. A. Gomaa, “Effects of microneedle length, density, insertion time and multiple applications on human skin barrier function: assessments by transepidermal water loss”, Toxicol. In Vitro 24(7), 1971–1978 (2010). http://dx.doi.org/10.1016/j.tiv.2010.08.012
- S. P. Davis, B. J. Landis, Z. H. Adams, M. G. Allen and M. R. Prausnitz, “Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force”, J. Biomech. 37(8), 1155–1163 (2004). http://dx.doi.org/10.1016/j.jbiomech.2003.12.010
- P. Ghosh, R. Pinninti, D. Hammell, K. Paudel and A. Stinchcomb, “Development of a codrug approach for sustained drug delivery across microneedle a treated skin”, J. Pharm. Sci. 102(5), 1458–1467 (2013). http://dx.doi.org/10.1002/jps.23469
- K. van der Maaden, W. Jiskoot and J. Bouwstra, “Microneedle technologies for (trans) dermal drug and vaccine delivery”, J. Controlled Release 161(2), 645–655 (2012). http://dx.doi.org/10.1016/j.jconrel.2012.01.042
- Y.-C. Kim, J.-H. Park and M. R. Prausnitz, “Microneedles for drug and vaccine delivery”, Adv. Drug Deliver. Rev. 64(14), 1547–1568 (2012). http://dx.doi.org/10.1016/j.addr.2012.04.005
- T. Fan, M. Li, X. Wu, M. Li and Y. Wu, “Preparation of thermoresponsive and pH-sensitivity polymer magnetic hydrogel nanospheres as anticancer drug carriers”, Colloids Surface B: Biointerface 88(2), 593–600 (2011). http://dx.doi.org/10.1016/j.colsurfb.2011.07.048
- C. J. Martin, C. J. Allendera, K. R. Braina, A. Morrisseyb and J. C. Birchall, “Low temperature fabrication of biodegradable sugar glass microneedles for transdermal drug delivery applications”, J. Controlled Release 158(1), 93–101 (2012). http://dx.doi.org/10.1016/j.jconrel.2011.10.024
- L. Liu, X. Tang, Y. Wang and S. Guo, “Smart gelation of chitosan solution in the presence of NaHCO3 for injectable drug delivery system”, Int. J. Pharm. 414(1–2), 6–15 (2011). http://dx.doi.org/10.1016/j.ijpharm.2011.04.052
- K. M. Gattás-Asfura, E. Weisman, F. M. Andreopoulos, M. Micic, B. Muller, S. Sirpal, S. M. Pham and R. M. Leblanc, “Nitrocinnamate-functionalized gelatin: synthesis and “smart” hydrogel formation via photo-cross-linking”, Biomacromolecules 6(3), 1503–1509 (2005). http://dx.doi.org/10.1021/bm049238w
- J. Kopeček, “Hydrogel biomaterials: a smart future?”, Biomaterials 28(34), 5185–5192 (2007). http://dx.doi.org/10.1016/j.biomaterials.2007.07.044
- I. Y. Galaev and B. Mattiasson, “‘Smart’polymers and what they could do in biotechnology and medicine”, Trends Biotechnol. 17(8), 335–340 (1999). http://dx.doi.org/10.1016/S0167-7799 (99)01345-1
- F. Wu, S. Yang, W. Yuan and T. Jin, “Challenges and strategies in developing microneedle patches for transdermal delivery of protein and peptide therapeutics”, Curr. Pharm. Biotechno. 13(7), 1292–1298 (2012). http://dx.doi.org/10.2174/138920112800624319
- J.-H. Park, M. G. Allen and M. R. Prausnitz, “Polymer microneedles for controlled-release drug delivery”, Pharm. Res. 23(5), 1008–1019 (2006). http://dx.doi.org/ 10.1007/s11095-006-0028-9
- L. Wei-Ze, H. Mei-Rong, Z. Jian-Ping, Z. Yong-Qiang, H. Bao-Hua, L. Ting and Z. Yong, “Super-short solid silicon microneedles for transdermal drug delivery applications”, Int. J. Pharm. 389(1), 122–129 (2010). http://dx.doi.org/10.1016/j.ijpharm.2010.01.024
- L. Daugimont, N. Baron, G. Vandermeulen, N. Pavselj, D. Miklavcic, M.-C. Jullien, G. Cabodevila, L. M. Mir and V. Préat, “Hollow microneedle arrays for intradermal drug delivery and DNA electroporation”, J. Membrane Biol. 236(1), 117–125 (2010). http://dx.doi.org/10.1007/s00232-010-9283-0
- Y. Qiu, G. Qin, S. Zhang, Y. Wu, B. Xu and Y. Gao, “Novel lyophilized hydrogel patches for convenient and effective administration of microneedle-mediated insulin delivery”, Int. J. Pharm. 437(1–2), 51–56 (2012). http://dx.doi.org/10.1016/j.ijpharm.2012.07.035
- L. Lin and A. P. Pisano, “Silicon-processed microneedles”, J. Microelectromech. S. 8(1), 78–84 (1999). http://dx.doi.org/10.1109/84.749406
- N. K. Brogden, M. Milewski, P. Ghosh, L. Hardi, L. J. Crofford and A. L. Stinchcomb, “Diclofenac delays micropore closure following microneedle treatment in human subjects”, J. Controlled Release 163(2), 220–229 (2012). http://dx.doi.org/10.1016/j.jconrel.2012.08.015
- H. S. Gill and M. R. Prausnitz, “Coated microneedles for transdermal delivery”, J. Controlled Release 117(2), 227–237 (2007). http://dx.doi.org/10.1016/j.jconrel.2006.10.017
- M. Pearton, C. Allender, K. Brain, A. Anstey, C. Gateley, N. Wilke, A. Morrissey and J. Birchall, “Gene delivery to the epidermal cells of human skin explants using microfabricated microneedles and hydrogel formulations”, Pharmaceut. Res. 25(2), 407–416 (2008). http://dx.doi.org/10.1007/s11095-007-9360-y
- A. K. Andrianov, A. Marin and D. P. DeCollibus, “Microneedles with intrinsic immunoadjuvant properties: microfabrication, protein stability, and modulated release”, Pharmaceut. Res. 28(1), 58–65 (2010). http://dx.doi.org/10.1007/s11095-010-0133-7
- A. Vrdoljak, M. G. McGrath, J. B. Carey, S. J. Draper, A. V. S. Hill, C. O’Mahony, A. M. Crean and A. C. Moore, “Coated microneedle arrays for transcutaneous delivery of live virus vaccines”, J. Controlled Release 159(1), 34–42 (2012). http://dx.doi.org/10.1016/j.jconrel.2011.12.026
- M. Pearton, V. Saller, S. A. Coulman, C. Gateley, A. V. Anstey, V. Zarnitsyn and J. C. Birchall, “Microneedle delivery of plasmid DNA to living human skin: formulation coating, skin insertion and gene expression”, J. Controlled Release 160(3), 561–569 (2012). http://dx.doi.org/10.1016/j.jconrel.2012.04.005
- M. Ashraf, S. Tayyaba, A. Nisar, N. Afzulpurkar, D. Bodhale, T. Lomas, A. Poyai and A. Tuantranont, “Design, fabrication and analysis of silicon hollow microneedles for transdermal drug delivery system for treatment of hemodynamic dysfunctions”, Cardiovasc. Eng. 10(3), 91–108 (2010). http://dx.doi.org/10.1007/s10558-010-9100-5
- M. Matteucci, M. Fanetti, M. Casella and F. Gramatica, “Poly vinyl alcohol re-usable masters for microneedle replication”, Microelectron. Eng. 86(4–6), 752 (2009). http://dx.doi.org/10.1016/j.mee.2009.01.068
- L. Y. Chu, S. O. Choi and M. R. Prausnitz, “Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs”, J. Pharm. Sci. 99(10), 4228–4238 (2010). http://dx.doi.org/10.1002/jps.22140
- X. Hong, L. Wei, F. Wu, Z. Wu, L. Chen, Z. Liu and W. Yuan, “Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine”, Drug Des. Dev. Ther. 7, 945–952 (2013). http://dx.doi.org/10.2147/DDDT.S44401
- J. W. Lee, M.-R. Han and J.-H. Park, “Polymer microneedles for transdermal drug delivery”, J. Drug Target 21(3), 211–223 (2013). http://dx.doi.org/10.3109/1061186X.2012.741136
- K. Migalska, D. I. J. Morrow, M. J. Garland, R. Thakur, A. D. Woolfson and R. F. Donnelly, “Laser-engineered dissolving microneedle arrays for transdermal macromolecular drug delivery”, Pharmaceut. Res. 28(8), 1919 (2011). http://dx.doi.org/10.1007/s11095-011-0419-4
- S. Aoyagi, H. Izumi, Y. Isono, M. Fukuda and H. Ogawa, “Laser fabrication of high aspect ratio thin holes on biodegradable polymer and its application to a microneedle”, Sensors and Actuators A: Physical. 139(1–2), 293–302 (2007). http://dx.doi.org/10.1016/j.sna.2006.11.022
- S. Liu, M.-n. Jin, Y.-s. Quan, F. Kamiyama, H. Katsumi, T. Sakane and A. Yamamoto, “The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin”, J. Control Release. 161(3), 933–941 (2012). http://dx.doi.org/10.1016/j.jconrel.2012.05.030
- Y. Ito, M. Hirono, K. Fukushima, N. Sugioka and K. Takada, “Two-layered dissolving microneedles formulated with intermediate-acting insulin”, Int. J. Pharm. 436(1–2), 387–393 (2012). http://dx.doi.org/10.1016/j.ijpharm.2012.06.047
- J.-H. Park, M. G. Allen and M. R. Prausnitz, “Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery”, J. Control Release 104(1), 51–66 (2005). http://dx.doi.org/10.1016/j.jconrel.2005.02.002
- J. W. Lee, J.-H. Park and M. R. Prausnitz, “Dissolving microneedles for transdermal drug delivery”, Biomaterials 29(13), 2113–2124 (2008). http://dx.doi.org/10.1016/j.biomaterials.2007.12.048
- M. C. Chen, S. F. Huang, K. Y. Lai and M. H. Ling, “Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination”, Biomaterials 34, 3077–3086 (2013). http://dx.doi.org/10.1016/j.biomaterials.2012.12.041
- M. H. Ling and M. C. Chen, “Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats”, Acta Biomaterialia 9, 8952–8961 (2013). http://dx.doi.org/10.1016/j.actbio.2013.06.029
- M.-C. Chen, M.-H. Ling, K.-Y. Lai and E. Pramudityo, “Chitosan Microneedle Patches for Sustained Transdermal Delivery of Macromolecules”, Biomacromolecules 13(12), 4022–4031 (2012). http://dx.doi.org/10.1021/bm301293d
- M. Kim, B. Jung and J.-H. Park, “Hydrogel swelling as a trigger to release biodegradable polymer microneedles in skin”, Biomaterials 33(2), 668–678 (2012). http://dx.doi.org/10.1016/j.biomaterials.2011.09.074
- L. Y. Chu and M. R. Prausnitz, “Separable arrowhead microneedles”, J. Control Release 149(3), 242–249 (2011). http://dx.doi.org/10.1016/j.jconrel.2010.10.033
- R. F. Donnelly, T. R. R. Singh, M. J. Garland, K. Migalska, R. Majithiya, C. M. McCrudden, P. L. Kole, T. M. T. Mahmood, H. O. McCarthy and A. D. Woolfson, “Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery”, Adv. Funct. Mater. 22(23), 4879–4890 (2012). http://dx.doi.org/10.1002/adfm.201200864
- R. F. Donnelly, R. Majithiya, T. R. R. Singh, D. I. J. Morrow, M. J. Garland, Y. K. Demir, K. Migalska, E. Ryan, D. Gillen, C. J. Scott and A. D. Woolfson, “Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique”, Pharmaceut. Res. 28(1), 41–57 (2010). http://dx.doi.org/10.1007/s11095-010-0169-8
- R. Boehm, P. Miller, S. Hayes, N. Monteiro-Riviere and R. Narayan, “Modification of microneedles using inkjet printing”, AIP advances 1(2), 022139–13 (2011). http://dx.doi.org/10.1063/1.3602461
- D. Yang, Y. Li and J. Nie, “Preparation of gelatin/PVA nanofibers and their potential application in controlled release of drugs”, Carbohyd. Polym. 69(3), 538–543 (2007). http://dx.doi.org/10.1016/j.carbpol.2007.01.008
- K. Masanori, J. Toguchida and M. Oka, “Preliminary study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus”, Biomaterials 24(4), 639–647 (2003). http://dx.doi.org/10.1016/S0142-9612(02)00378-2
- S. Yang, Y. Feng, L. Zhang, N. Chen, W. Yuan and T. Jin, “A scalable fabrication process of polymer microneedles”, Int. J. Nanomed. 7, 1415 (2012). http://dx.doi.org/10.2147/IJN.S28511
- J. Su, J. Mazzeo, N. Subbarao and T. Jin, “Conference report: pharmaceutical development of biologics: fundamentals, challenges and recent advances”, Therapeutic Delivery 2(7), 865–871 (2011). http://dx.doi.org/10.4155/tde.11.58
- Y. K. Demir, Z. Akan and O. Kerimoglu, “Sodium alginate microneedle arrays mediate the transdermal delivery of bovine serum albumin”, PloS one 8(5), e63819 (2013). http://dx.doi.org/10.1371/journal.pone.0063819
- Y. K. Demir, Z. Akan and O. Kerimoglu, “Characterization of polymeric microneedle arrays for transdermal drug delivery”, PloS one 8(10), e77289 (2013). http://dx.doi.org/10.1371/journal.pone.0077289
- M. J. Garland, E. Caffarel-Salvador, K. Migalska, A. D. Woolfson and R. F. Donnelly, “Dissolving polymeric microneedle arrays for electrically assisted transdermal drug delivery”, J. Control Release 159(1), 52–59 (2012). http://dx.doi.org/10.1016/j.jconrel.2012.01.003
- H. Chen, H. Zhu, J. Zheng, D. Mou, J. Wan, J. Zhang, T. Shi, Y. Zhao, H. Xu and X. Yang, “Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin”, J. Control Release 139(1), 63–72 (2009). http://dx.doi.org/10.1016/j.jconrel.2009.05.031
- M. G Nava-Arzaluz, I. Calderon-Lojero, D. Quintanar-Guerrero, R. Villalobos-Garcia and A. Ganem-Quintanar, “Microneedles as transdermal delivery systems: combination with other enhancing strategies”, Current Drug Delivery 9(1), 57–73 (2012). http://dx.doi.org/10.2174/156720112798376078
- A.-R. Denet, R. Vanbever and V. Préat, “Skin electroporation for transdermal and topical delivery”, Adv. Drug Deliver. Rev. 56(5), 659–674 (2004). http://dx.doi.org/10.1016/j.addr.2003.10.027
- V. Kumar, “Modulated iontophoretic delivery of small and large molecules through microchannels”, Int. J. Pharm. 434(1–2), 106 (2012). http://dx.doi.org/10.1016/j.ijpharm.2012.05.030
- C.-J. Ke, Y.-J. Lin, Y.-C. Hu, W.-L. Chiang, K.-J. Chen, W.-C. Yang, H.-L. Liu, C.-C. Fu and H.-W. Sung, “Multidrug release based on microneedle arrays filled with pH-responsive PLGA hollow microspheres”, Biomaterials 33(20), 5156–5165 (2012). http://dx.doi.org/10.1016/j.biomaterials.2012.03.056
- X.-P. Fu, “On the Influence of the Psychological Shift of the Scholars in Mid-Ming Dynasty on Zisha Teapot Prosperity”, Journal of Chengdu University of Technology (Social Sciences) 16(1), 11–15 (2008).
- J. Wu, T. Hou, M. Zhang, Q. Li, J. Wu, J. Li and Z. Deng, “An analysis of the chemical composition, performance and structure of China Yixing Zisha pottery from 1573 AD to 1911 AD”, Ceram. Int. 39(3), 2589–2595 (2013). http://dx.doi.org/10.1016/j.ceramint.2012.09.021
- J. Sun and M. L. Ruan, “Microstructure and properties of Yixing Zisha ware”, China Ceramics 4, 21–25 (1993).
- R. F. Donnelly, T. R. R. Singh, A. Z. Alkilani, M. T. C. McCrudden, S. O’Neill, C. O’Mahony, K. Armstrong, N. McLoone, P. Kole and A. D. Woolfson, “Hydrogel-forming microneedle arrays exhibit antimicrobial properties: Potential for enhanced patient safety”, Int. J. Pharm. 451(1–2), 76–91 (2013). http://dx.doi.org/10.1016/j.ijpharm.2013.04.045
- R. F. Donnelly, T. R. R. Singh, M. J. Garland, K. Migalska, R. Majithiya, C. M. McCrudden, P. L. Kole, T. M. Mahmood, H. O. McCarthy and A. D. Woolfson, “Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery”, Adv. Funct. Mater. 22(23), 4879–4890 (2012). http://dx.doi.org/10.1002/adfm.201200864
- K. Juntanon, S. Niamlang, R. Rujiravanit and A. Sirivat, “Electrically controlled release of sulfosalicylic acid from crosslinked poly(vinyl alcohol) hydrogel”, Int. J. Pharm. 356(1–2), 1–11 (2008). http://dx.doi.org/10.1016/j.ijpharm.2007.12.023
- Y. Qiu, G. Qin, S. Zhang, Y. Wu, B. Xu and Y. Gao, “Novel lyophilized hydrogel patches for convenient and effective administration of microneedle-mediated insulin delivery”, Int. J. Pharm. 437(122), 51–56 (2012). http://dx.doi.org/10.1016/j.ijpharm.2012.07.035
References
M. R. Prausnitz and R. Langer, “Transdermal drug delivery”, Nat. Biotechnol. 26(11), 1261–1268 (2008). http://dx.doi.org/10.1038/nbt.1504
R. F. Donnelly, T. R. R. Singh and A. D. Woolfson, “Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety”, Drug Deliv. 17(4), 187–207 (2010). http://dx.doi.org/10.3109/10717541003667798
Y. A. Gomaa, “Effects of microneedle length, density, insertion time and multiple applications on human skin barrier function: assessments by transepidermal water loss”, Toxicol. In Vitro 24(7), 1971–1978 (2010). http://dx.doi.org/10.1016/j.tiv.2010.08.012
S. P. Davis, B. J. Landis, Z. H. Adams, M. G. Allen and M. R. Prausnitz, “Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force”, J. Biomech. 37(8), 1155–1163 (2004). http://dx.doi.org/10.1016/j.jbiomech.2003.12.010
P. Ghosh, R. Pinninti, D. Hammell, K. Paudel and A. Stinchcomb, “Development of a codrug approach for sustained drug delivery across microneedle a treated skin”, J. Pharm. Sci. 102(5), 1458–1467 (2013). http://dx.doi.org/10.1002/jps.23469
K. van der Maaden, W. Jiskoot and J. Bouwstra, “Microneedle technologies for (trans) dermal drug and vaccine delivery”, J. Controlled Release 161(2), 645–655 (2012). http://dx.doi.org/10.1016/j.jconrel.2012.01.042
Y.-C. Kim, J.-H. Park and M. R. Prausnitz, “Microneedles for drug and vaccine delivery”, Adv. Drug Deliver. Rev. 64(14), 1547–1568 (2012). http://dx.doi.org/10.1016/j.addr.2012.04.005
T. Fan, M. Li, X. Wu, M. Li and Y. Wu, “Preparation of thermoresponsive and pH-sensitivity polymer magnetic hydrogel nanospheres as anticancer drug carriers”, Colloids Surface B: Biointerface 88(2), 593–600 (2011). http://dx.doi.org/10.1016/j.colsurfb.2011.07.048
C. J. Martin, C. J. Allendera, K. R. Braina, A. Morrisseyb and J. C. Birchall, “Low temperature fabrication of biodegradable sugar glass microneedles for transdermal drug delivery applications”, J. Controlled Release 158(1), 93–101 (2012). http://dx.doi.org/10.1016/j.jconrel.2011.10.024
L. Liu, X. Tang, Y. Wang and S. Guo, “Smart gelation of chitosan solution in the presence of NaHCO3 for injectable drug delivery system”, Int. J. Pharm. 414(1–2), 6–15 (2011). http://dx.doi.org/10.1016/j.ijpharm.2011.04.052
K. M. Gattás-Asfura, E. Weisman, F. M. Andreopoulos, M. Micic, B. Muller, S. Sirpal, S. M. Pham and R. M. Leblanc, “Nitrocinnamate-functionalized gelatin: synthesis and “smart” hydrogel formation via photo-cross-linking”, Biomacromolecules 6(3), 1503–1509 (2005). http://dx.doi.org/10.1021/bm049238w
J. Kopeček, “Hydrogel biomaterials: a smart future?”, Biomaterials 28(34), 5185–5192 (2007). http://dx.doi.org/10.1016/j.biomaterials.2007.07.044
I. Y. Galaev and B. Mattiasson, “‘Smart’polymers and what they could do in biotechnology and medicine”, Trends Biotechnol. 17(8), 335–340 (1999). http://dx.doi.org/10.1016/S0167-7799 (99)01345-1
F. Wu, S. Yang, W. Yuan and T. Jin, “Challenges and strategies in developing microneedle patches for transdermal delivery of protein and peptide therapeutics”, Curr. Pharm. Biotechno. 13(7), 1292–1298 (2012). http://dx.doi.org/10.2174/138920112800624319
J.-H. Park, M. G. Allen and M. R. Prausnitz, “Polymer microneedles for controlled-release drug delivery”, Pharm. Res. 23(5), 1008–1019 (2006). http://dx.doi.org/ 10.1007/s11095-006-0028-9
L. Wei-Ze, H. Mei-Rong, Z. Jian-Ping, Z. Yong-Qiang, H. Bao-Hua, L. Ting and Z. Yong, “Super-short solid silicon microneedles for transdermal drug delivery applications”, Int. J. Pharm. 389(1), 122–129 (2010). http://dx.doi.org/10.1016/j.ijpharm.2010.01.024
L. Daugimont, N. Baron, G. Vandermeulen, N. Pavselj, D. Miklavcic, M.-C. Jullien, G. Cabodevila, L. M. Mir and V. Préat, “Hollow microneedle arrays for intradermal drug delivery and DNA electroporation”, J. Membrane Biol. 236(1), 117–125 (2010). http://dx.doi.org/10.1007/s00232-010-9283-0
Y. Qiu, G. Qin, S. Zhang, Y. Wu, B. Xu and Y. Gao, “Novel lyophilized hydrogel patches for convenient and effective administration of microneedle-mediated insulin delivery”, Int. J. Pharm. 437(1–2), 51–56 (2012). http://dx.doi.org/10.1016/j.ijpharm.2012.07.035
L. Lin and A. P. Pisano, “Silicon-processed microneedles”, J. Microelectromech. S. 8(1), 78–84 (1999). http://dx.doi.org/10.1109/84.749406
N. K. Brogden, M. Milewski, P. Ghosh, L. Hardi, L. J. Crofford and A. L. Stinchcomb, “Diclofenac delays micropore closure following microneedle treatment in human subjects”, J. Controlled Release 163(2), 220–229 (2012). http://dx.doi.org/10.1016/j.jconrel.2012.08.015
H. S. Gill and M. R. Prausnitz, “Coated microneedles for transdermal delivery”, J. Controlled Release 117(2), 227–237 (2007). http://dx.doi.org/10.1016/j.jconrel.2006.10.017
M. Pearton, C. Allender, K. Brain, A. Anstey, C. Gateley, N. Wilke, A. Morrissey and J. Birchall, “Gene delivery to the epidermal cells of human skin explants using microfabricated microneedles and hydrogel formulations”, Pharmaceut. Res. 25(2), 407–416 (2008). http://dx.doi.org/10.1007/s11095-007-9360-y
A. K. Andrianov, A. Marin and D. P. DeCollibus, “Microneedles with intrinsic immunoadjuvant properties: microfabrication, protein stability, and modulated release”, Pharmaceut. Res. 28(1), 58–65 (2010). http://dx.doi.org/10.1007/s11095-010-0133-7
A. Vrdoljak, M. G. McGrath, J. B. Carey, S. J. Draper, A. V. S. Hill, C. O’Mahony, A. M. Crean and A. C. Moore, “Coated microneedle arrays for transcutaneous delivery of live virus vaccines”, J. Controlled Release 159(1), 34–42 (2012). http://dx.doi.org/10.1016/j.jconrel.2011.12.026
M. Pearton, V. Saller, S. A. Coulman, C. Gateley, A. V. Anstey, V. Zarnitsyn and J. C. Birchall, “Microneedle delivery of plasmid DNA to living human skin: formulation coating, skin insertion and gene expression”, J. Controlled Release 160(3), 561–569 (2012). http://dx.doi.org/10.1016/j.jconrel.2012.04.005
M. Ashraf, S. Tayyaba, A. Nisar, N. Afzulpurkar, D. Bodhale, T. Lomas, A. Poyai and A. Tuantranont, “Design, fabrication and analysis of silicon hollow microneedles for transdermal drug delivery system for treatment of hemodynamic dysfunctions”, Cardiovasc. Eng. 10(3), 91–108 (2010). http://dx.doi.org/10.1007/s10558-010-9100-5
M. Matteucci, M. Fanetti, M. Casella and F. Gramatica, “Poly vinyl alcohol re-usable masters for microneedle replication”, Microelectron. Eng. 86(4–6), 752 (2009). http://dx.doi.org/10.1016/j.mee.2009.01.068
L. Y. Chu, S. O. Choi and M. R. Prausnitz, “Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs”, J. Pharm. Sci. 99(10), 4228–4238 (2010). http://dx.doi.org/10.1002/jps.22140
X. Hong, L. Wei, F. Wu, Z. Wu, L. Chen, Z. Liu and W. Yuan, “Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine”, Drug Des. Dev. Ther. 7, 945–952 (2013). http://dx.doi.org/10.2147/DDDT.S44401
J. W. Lee, M.-R. Han and J.-H. Park, “Polymer microneedles for transdermal drug delivery”, J. Drug Target 21(3), 211–223 (2013). http://dx.doi.org/10.3109/1061186X.2012.741136
K. Migalska, D. I. J. Morrow, M. J. Garland, R. Thakur, A. D. Woolfson and R. F. Donnelly, “Laser-engineered dissolving microneedle arrays for transdermal macromolecular drug delivery”, Pharmaceut. Res. 28(8), 1919 (2011). http://dx.doi.org/10.1007/s11095-011-0419-4
S. Aoyagi, H. Izumi, Y. Isono, M. Fukuda and H. Ogawa, “Laser fabrication of high aspect ratio thin holes on biodegradable polymer and its application to a microneedle”, Sensors and Actuators A: Physical. 139(1–2), 293–302 (2007). http://dx.doi.org/10.1016/j.sna.2006.11.022
S. Liu, M.-n. Jin, Y.-s. Quan, F. Kamiyama, H. Katsumi, T. Sakane and A. Yamamoto, “The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin”, J. Control Release. 161(3), 933–941 (2012). http://dx.doi.org/10.1016/j.jconrel.2012.05.030
Y. Ito, M. Hirono, K. Fukushima, N. Sugioka and K. Takada, “Two-layered dissolving microneedles formulated with intermediate-acting insulin”, Int. J. Pharm. 436(1–2), 387–393 (2012). http://dx.doi.org/10.1016/j.ijpharm.2012.06.047
J.-H. Park, M. G. Allen and M. R. Prausnitz, “Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery”, J. Control Release 104(1), 51–66 (2005). http://dx.doi.org/10.1016/j.jconrel.2005.02.002
J. W. Lee, J.-H. Park and M. R. Prausnitz, “Dissolving microneedles for transdermal drug delivery”, Biomaterials 29(13), 2113–2124 (2008). http://dx.doi.org/10.1016/j.biomaterials.2007.12.048
M. C. Chen, S. F. Huang, K. Y. Lai and M. H. Ling, “Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination”, Biomaterials 34, 3077–3086 (2013). http://dx.doi.org/10.1016/j.biomaterials.2012.12.041
M. H. Ling and M. C. Chen, “Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats”, Acta Biomaterialia 9, 8952–8961 (2013). http://dx.doi.org/10.1016/j.actbio.2013.06.029
M.-C. Chen, M.-H. Ling, K.-Y. Lai and E. Pramudityo, “Chitosan Microneedle Patches for Sustained Transdermal Delivery of Macromolecules”, Biomacromolecules 13(12), 4022–4031 (2012). http://dx.doi.org/10.1021/bm301293d
M. Kim, B. Jung and J.-H. Park, “Hydrogel swelling as a trigger to release biodegradable polymer microneedles in skin”, Biomaterials 33(2), 668–678 (2012). http://dx.doi.org/10.1016/j.biomaterials.2011.09.074
L. Y. Chu and M. R. Prausnitz, “Separable arrowhead microneedles”, J. Control Release 149(3), 242–249 (2011). http://dx.doi.org/10.1016/j.jconrel.2010.10.033
R. F. Donnelly, T. R. R. Singh, M. J. Garland, K. Migalska, R. Majithiya, C. M. McCrudden, P. L. Kole, T. M. T. Mahmood, H. O. McCarthy and A. D. Woolfson, “Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery”, Adv. Funct. Mater. 22(23), 4879–4890 (2012). http://dx.doi.org/10.1002/adfm.201200864
R. F. Donnelly, R. Majithiya, T. R. R. Singh, D. I. J. Morrow, M. J. Garland, Y. K. Demir, K. Migalska, E. Ryan, D. Gillen, C. J. Scott and A. D. Woolfson, “Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique”, Pharmaceut. Res. 28(1), 41–57 (2010). http://dx.doi.org/10.1007/s11095-010-0169-8
R. Boehm, P. Miller, S. Hayes, N. Monteiro-Riviere and R. Narayan, “Modification of microneedles using inkjet printing”, AIP advances 1(2), 022139–13 (2011). http://dx.doi.org/10.1063/1.3602461
D. Yang, Y. Li and J. Nie, “Preparation of gelatin/PVA nanofibers and their potential application in controlled release of drugs”, Carbohyd. Polym. 69(3), 538–543 (2007). http://dx.doi.org/10.1016/j.carbpol.2007.01.008
K. Masanori, J. Toguchida and M. Oka, “Preliminary study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus”, Biomaterials 24(4), 639–647 (2003). http://dx.doi.org/10.1016/S0142-9612(02)00378-2
S. Yang, Y. Feng, L. Zhang, N. Chen, W. Yuan and T. Jin, “A scalable fabrication process of polymer microneedles”, Int. J. Nanomed. 7, 1415 (2012). http://dx.doi.org/10.2147/IJN.S28511
J. Su, J. Mazzeo, N. Subbarao and T. Jin, “Conference report: pharmaceutical development of biologics: fundamentals, challenges and recent advances”, Therapeutic Delivery 2(7), 865–871 (2011). http://dx.doi.org/10.4155/tde.11.58
Y. K. Demir, Z. Akan and O. Kerimoglu, “Sodium alginate microneedle arrays mediate the transdermal delivery of bovine serum albumin”, PloS one 8(5), e63819 (2013). http://dx.doi.org/10.1371/journal.pone.0063819
Y. K. Demir, Z. Akan and O. Kerimoglu, “Characterization of polymeric microneedle arrays for transdermal drug delivery”, PloS one 8(10), e77289 (2013). http://dx.doi.org/10.1371/journal.pone.0077289
M. J. Garland, E. Caffarel-Salvador, K. Migalska, A. D. Woolfson and R. F. Donnelly, “Dissolving polymeric microneedle arrays for electrically assisted transdermal drug delivery”, J. Control Release 159(1), 52–59 (2012). http://dx.doi.org/10.1016/j.jconrel.2012.01.003
H. Chen, H. Zhu, J. Zheng, D. Mou, J. Wan, J. Zhang, T. Shi, Y. Zhao, H. Xu and X. Yang, “Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin”, J. Control Release 139(1), 63–72 (2009). http://dx.doi.org/10.1016/j.jconrel.2009.05.031
M. G Nava-Arzaluz, I. Calderon-Lojero, D. Quintanar-Guerrero, R. Villalobos-Garcia and A. Ganem-Quintanar, “Microneedles as transdermal delivery systems: combination with other enhancing strategies”, Current Drug Delivery 9(1), 57–73 (2012). http://dx.doi.org/10.2174/156720112798376078
A.-R. Denet, R. Vanbever and V. Préat, “Skin electroporation for transdermal and topical delivery”, Adv. Drug Deliver. Rev. 56(5), 659–674 (2004). http://dx.doi.org/10.1016/j.addr.2003.10.027
V. Kumar, “Modulated iontophoretic delivery of small and large molecules through microchannels”, Int. J. Pharm. 434(1–2), 106 (2012). http://dx.doi.org/10.1016/j.ijpharm.2012.05.030
C.-J. Ke, Y.-J. Lin, Y.-C. Hu, W.-L. Chiang, K.-J. Chen, W.-C. Yang, H.-L. Liu, C.-C. Fu and H.-W. Sung, “Multidrug release based on microneedle arrays filled with pH-responsive PLGA hollow microspheres”, Biomaterials 33(20), 5156–5165 (2012). http://dx.doi.org/10.1016/j.biomaterials.2012.03.056
X.-P. Fu, “On the Influence of the Psychological Shift of the Scholars in Mid-Ming Dynasty on Zisha Teapot Prosperity”, Journal of Chengdu University of Technology (Social Sciences) 16(1), 11–15 (2008).
J. Wu, T. Hou, M. Zhang, Q. Li, J. Wu, J. Li and Z. Deng, “An analysis of the chemical composition, performance and structure of China Yixing Zisha pottery from 1573 AD to 1911 AD”, Ceram. Int. 39(3), 2589–2595 (2013). http://dx.doi.org/10.1016/j.ceramint.2012.09.021
J. Sun and M. L. Ruan, “Microstructure and properties of Yixing Zisha ware”, China Ceramics 4, 21–25 (1993).
R. F. Donnelly, T. R. R. Singh, A. Z. Alkilani, M. T. C. McCrudden, S. O’Neill, C. O’Mahony, K. Armstrong, N. McLoone, P. Kole and A. D. Woolfson, “Hydrogel-forming microneedle arrays exhibit antimicrobial properties: Potential for enhanced patient safety”, Int. J. Pharm. 451(1–2), 76–91 (2013). http://dx.doi.org/10.1016/j.ijpharm.2013.04.045
R. F. Donnelly, T. R. R. Singh, M. J. Garland, K. Migalska, R. Majithiya, C. M. McCrudden, P. L. Kole, T. M. Mahmood, H. O. McCarthy and A. D. Woolfson, “Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery”, Adv. Funct. Mater. 22(23), 4879–4890 (2012). http://dx.doi.org/10.1002/adfm.201200864
K. Juntanon, S. Niamlang, R. Rujiravanit and A. Sirivat, “Electrically controlled release of sulfosalicylic acid from crosslinked poly(vinyl alcohol) hydrogel”, Int. J. Pharm. 356(1–2), 1–11 (2008). http://dx.doi.org/10.1016/j.ijpharm.2007.12.023
Y. Qiu, G. Qin, S. Zhang, Y. Wu, B. Xu and Y. Gao, “Novel lyophilized hydrogel patches for convenient and effective administration of microneedle-mediated insulin delivery”, Int. J. Pharm. 437(122), 51–56 (2012). http://dx.doi.org/10.1016/j.ijpharm.2012.07.035