RhB Adsorption Performance of Magnetic Adsorbent Fe3O4/RGO Composite and Its Regeneration through A Fenton-like Reaction
Corresponding Author: Mingce Long
Nano-Micro Letters,
Vol. 6 No. 2 (2014), Article Number: 125-135
Abstract
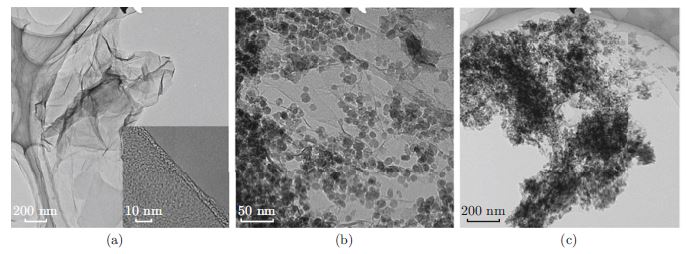
Adsorption is one of the most effective technologies in the treatment of colored matter containing wastewater. Graphene related composites display potential to be an effective adsorbent. However, the adsorption mechanism and their regeneration approach are still demanding more efforts. An effective magnetically separable absorbent, Fe3O4 and reduced graphene oxide (RGO) composite has been prepared by an in situ coprecipitation and reduction method. According to the characterizations of TEM, XRD, XPS, Raman spectra and BET analyses, Fe3O4 nanoparticles in sizes of 10–20 nm are well dispersed over the RGO nanosheets, resulting in a highest specific area of 296.2 m2/g. The rhodamine B adsorption mechanism on the composites was investigated by the adsorption kinetics and isotherms. The isotherms are fitting better by Langmuir model, and the adsorption kinetic rates depend much on the chemical components of RGO. Compared to active carbon, the composite shows 3.7 times higher adsorption capacity and thirty times faster adsorption rates. Furthermore, with Fe3O4 nanoparticles as the in situ catalysts, the adsorption performance of composites can be restored by carrying out a Fenton-like reaction, which could be a promising regeneration way for the adsorbents in the organic pollutant removal of wastewater.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- R. Sanghi and P. Verma, “Decolorisation of aqueous dye solutions by low-cost adsorbents: a review”, Color. Technol. 129(2), 85–108 (2013). http://dx.doi.org/10.1111/cote.12019
- A. Dqbrowski, “Adsorption — from theory to practice”, Adv. Colloid Interface Sci. 93(1–3), 135–224 (2001). http://dx.doi.org/10.1016/S0001-8686(00)00082-8
- J. C. Lazo-Cannata, A. Nieto-Márquez, A. Jacoby, A. L. Paredes-Doig, A. Romero, M. R. Sun-Kou and J. L. Valverde, “Adsorption of phenol and nitrophenols by carbon nanospheres: Effect of pH and ionic strength”, Sep. Purif. Technol. 80(2), 217–224 (2011). http://dx.doi.org/10.1016/j.seppur.2011.04.029
- R. Liu, W. Gong, H. Lan, T. Yang, H. Liu and J. Qu, “Simultaneous removal of arsenate and fluoride by iron and aluminum binary oxide: Competitive adsorption effects”, Sep. Purif. Technol. 92, 100–105 (2012). http://dx.doi.org/10.1016/j.seppur.2012.03.020
- X. Hu, B. Liu, Y. Deng, H. Chen, S. Luo, C. Sun, P. Yang and S. Yang, “Adsorption and heterogeneous Fenton degradation of 17α-methyltestosterone on nano Fe3O4/MWCNTs in aqueous solution”, Appl. Catal. B: Environ. 107(3-4), 274–283 (2011). http://dx.doi.org/10.1016/j.apcatb.2011.07.025
- S. Tang, N. Lu, J. Li and Y. Wu, “Design and application of an up-scaled dielectric barrier discharge plasma reactor for regeneration of phenolsaturated granular activated carbon”, Sep. Purif. Technol. 95, 73–79 (2012). http://dx.doi.org/10.1016/j.seppur.2012.05.002
- V. K. K. Upadhyayula, S. Deng, M. C. Mitchell and G. B. Smith, “Application of carbon nanotube technology for removal of contaminants in drinking water: a review”, Sci. Total Environ. 408(1), 1–13 (2009). http://dx.doi.org/10.1016/j.scitotenv.2009.09.027
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, “Graphene and graphene oxide: synthesis, properties, and applications”, Adv. Mater. 22(35), 3906–3924 (2010). http://dx.doi.org/10.1002/adma.201001068
- J. Kim, L. J. Cote and J. Huang, “Two dimensional soft material: new faces of graphene oxide”, Acc. Chem. Res. 45(8), 1356–1364 (2012). http://dx.doi.org/10.1021/ar300047s
- S. Wang, H. Sun, H. M. Ang and M. O. Tadé, “Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials”, Chem. Eng. J. 226, 336–347 (2013). http://dx.doi.org/10.1016/j.cej.2013.04.070
- K. C. Kemp, H. Seema, M. Saleh, N. H. Le, K. Mahesh, V. Chandra and K. S. Kim, “Environmental applications using graphene composites: water remediation and gas adsorption”, Nanoscale 5(8), 3149–71 (2013). http://dx.doi.org/10.1039/c3nr33708a
- Y. Zhi, G. Rungang, H. Nantao, C. Jing, C. Yingwu, Z. Liying, W. Hao, K. Eric Siu-Wai and Z. Yafei, “The prospective 2D graphene nanosheets: preparation, functionalization and applications”, Nano-Micro Lett. 4(1), 1–9 (2012). http://dx.doi.org/10.3786/nml.v4i1.p1-9
- J. Kim, L. J. Cote, F. Kim, W. Yuan, K. R. Shull and J. Huang, “Graphene oxide sheets at interfaces”, J. Am. Chem. Soc. 132(23), 8180–8186 (2010). http://dx.doi.org/10.1021/ja102777p
- Z. Liu, J. T. Robinson, X. Sun and H. Dai, “PEGylated nanographene oxide for delivery of water-Insoluble cancer drugs”, J. Am. Chem. Soc. 130(33), 10876–10877 (2008). http://dx.doi.org/10.1021/ja803688x
- G. Zhao, T. Wen, C. Chen and X. Wang, “Synthesis of graphene-based nanomaterials and their application in energy-related and environmental-related areas”, RSC Adv. 2(25), 9286–9303 (2012). http://dx.doi.org/10.1039/c2ra20990j
- L. Wan, M. Long, D. Zhou, L. Zhang and W. Cai, “Preparation and characterization of freestanding hierarchical porous TiO2 monolith modified with graphene oxide”, Nano-Micro Lett. 4(2), 90–97 (2012). http://dx.doi.org/dx.doi.org/10.3786/nml.v4i2.p90-97
- C. T. Yavuz, J. T. Mayo, W. W. Yu, A. Prakash, J. C. Falkner, S. Yean, L. Cong, H. J. Shipley, A. Kan, M. Tomson, D. Natelson and V. L. Colvin, “Low-field magnetic separation of monodisperse Fe3O4 nanocrystals”, Science 314(5801), 964–967 (2006). http://dx.doi.org/10.1126/science.1131475
- M. Tayyebeh, A. Abbas, Z. Mohammad Ali, A. Mazaher and K. Nadia, “Application of modified silica coated magnetite nanoparticles for removal of iodine from water samples”, Nano-Micro Lett. 4(1), 57–63 (2012). http://dx.doi.org/10.3786/nml.v4i1.p57-63
- H. Sun, L. Cao and L. Lu, “Magnetite/reduced graphene oxide nanocomposites: one step solvothermal synthesis and use as a novel platform for removal of dye pollutants”, Nano Res. 4(6), 550–562 (2011). http://dx.doi.org/10.1007/s12274-011-0111-3
- Y. Huang and A. A. Keller, “Magnetic nanoparticle adsorbents for emerging organic contaminants”, ACS Sustainable Chem. Eng. 1(1), 731–736 (2013). http://dx.doi.org/10.1021/sc400047q
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, “Intrinsic peroxidase-like activity of ferromagnetic nanoparticles”, Nat. Nanotechnol. 2(9), 577–83 (2007). http://dx.doi.org/10.1038/nnano.2007.260
- J. Zhang, J. Zhuang, L. Gao, Y. Zhang, N. Gu, J. Feng, D. Yang, J. Zhu and X. Yan, “Decomposing phenol by the hidden talent of ferromagnetic nanoparticles”, Chemosphere 73(9), 1524–1528 (2008). http://dx.doi.org/10.1016/j.chemosphere.2008.05.050
- V. Chandra, J. Park, Y. Chun, J. W. Lee, I. C. Hwang and K. S. Kim, “Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal”, ACS Nano 4(7), 3979–3986. (2010). http://dx.doi.org/10.1021/nn1008897
- Z. Geng, Y. Lin, X. Yu, Q. Shen, L. Ma, Z. Li, N. Pan and X. Wang, “Highly efficient dye adsorption and removal: a functional hybrid of reduced graphene oxide-Fe3O4 nanoparticles as an easily regenerative adsorbent”, J. Mater. Chem. 22 (8), 3527–3535 (2012). http://dx.doi.org/10.1039/c2jm15544c
- F. He, J. Fan, D. Ma, L. Zhang, C. Leung and H. L. Chan, “The attachment of Fe3O4nanoparticles to graphene oxide by covalent bonding”, Carbon 48(11), 3139–3144 (2010). http://dx.doi.org/10.1016/j.carbon.2010.04.052
- M. Liu, C. Chen, J. Hu, X. Wu and X. Wang, “Synthesis of magnetite/graphene oxide composite and application for cobalt(II) removal”, J. Phys. Chem. C. 115(51), 25234–25240 (2011). http://dx.doi.org/10.1021/jp208575m
- G. Xie, P. Xi, H. Liu, F. Chen, L. Huang, Y. Shi, F. Hou, Z. Zeng, C. Shao and J. Wang, “A facile chemical method to produce superparamagnetic graphene oxide-Fe3O4 hybrid composite and its application in the removal of dyes from aqueous solution”, J. Mater. Chem. 22(3), 1033–1039 (2012). http://dx.doi.org/10.1039/c1jm13433g
- X. Zhou, Y. Zhang, C. Wang, X. Wu, Y. Yang, B. Zheng, H. Wu, S. Guo and J. Zhang, “Photo-Fenton reaction of graphene oxide: A new strategy to prepare graphene quantum dots for DNA cleavage”, ACS Nano 6(8), 6592–6599 (2012). http://dx.doi.org/10.1021/nn301629v
- S. Q. Liu, B. Xiao, L. R. Feng, S. S. Zhou, Z. G. Chen, C. B. Liu, F. Chen, Z. Y. Wu, N. Xu, W. C. Oh and Z. D. Meng, “Graphene oxide enhances the Fentonlike photocatalytic activity of nickel ferrite for degradation of dyes under visible light irradiation”, Carbon. 64, 197–206 (2013). http://dx.doi.org/10.1016/j.carbon.2013.07.052
- Y. C. Lee, S. J. Chang, M. H. Choi, T. J. Jeon, T. Ryu and Y. S. Huh, “Self-assembled graphene oxide with organo-building blocks of Fe-aminoclay for heterogeneous Fenton-like reaction at near-neutral pH: a batch experiment”, Appl. Catal. B-Environ. 142, 494–503 (2013). http://dx.doi.org/10.1016/j.apcatb.2013.05.066
- J. J. An, L. H. Zhu, N. Wang, Z. Song, Z. Y. Yang, D. Y. Du and H. Q. Tang, “Photo-Fenton like degradation of tetrabromobisphenol A with graphene-BiFeO3 composite as a catalyst”, Chem. Eng. J. 219, 225–237 (2013). http://dx.doi.org/10.1016/j.cej.2013.01.013
- W. S. Hummers and R. E. Offeman, “Preparation of graphitic oxide”, J. Am. Chem. Soc. 80(6), 1339–1339 (1958). http://dx.doi.org/10.1021/ja01539a017
- C. Chen, W. Cai, M. Long, B. Zhou, Y. Wu, D. Wu and Y. Feng, “Synthesis of visible light responsive graphene oxide/TiO2 composites with p/n heterojunction”, ACS Nano 4(11), 6425–6432 (2010). http://dx.doi.org/10.1021/nn102130m
- G. Wang, X. Shen, B. Wang, J. Yao and J. Park, “Synthesis and characterisation of hydrophilic and organophilic graphene nanosheets.”, Carbon 47(5), 1359–1364 (2009). http://dx.doi.org/10.1016/j.carbon.2009.01.027
- Z. J. Fan, W. Kai, J. Yan, T. Wei, L. J. Zhi, J. Feng, Y. Ren, L. P. Song and F. Wei, “Facile synthesis of graphene nanosheets via Fe reduction of exfoliated graphite oxide”, ACS Nano 5(1), 191–198 (2011). http://dx.doi.org/10.1021/nn102339t
- P. Wang, J. Wang, T. Ming, X. Wang, H. Yu, J. Yu, Y. Wang and M. Lei, “Dye-sensitization-induced visible-light reduction of graphene oxide for the enhanced TiO2 photocatalytic performance”, ACS Appl. Mater. Interfaces 5(8), 2924–2929 (2013). http://dx.doi.org/10.1021/am4008566
- Y. Liu, W. Jiang, Y. Wang, X. J. Zhang, D. Song and F. S. Li, “Synthesis of Fe3O4/CNTs magnetic nanocomposites at the liquid-liquid interface using oleate as surfactant and reactant”, J. Magn. Magn. Mater. 321(5), 408–412 (2009). http://dx.doi.org/10.1016/j.jmmm.2008.09.039
- X. Gou, G. Wang, J. Park, H. Liu and J. Yang, “Monodisperse hematite porous nanospheres: synthesis, characterization, and applications for gas sensors”, Nanotechnol. 19(12), 125606 (2008). http://dx.doi.org/10.1088/0957-4484/19/12/125606
- O. N. Shebanova and P. Lazor, “Raman study of magnetite (Fe3O4): laser-induced thermal effects and oxidation”, J. Raman Spectrosc. 34(11), 845–852 (2003). http://dx.doi.org/10.1002/jrs.1056
- M. Long, Y. Qin, C. Chen, X. Guo, B. Tan and W. Cai, “Origin of visible light photoactivity of RGO/TiO2 by in situ hydrothermal growth of under-grown TiO2 with graphene oxide”, J. Phys. Chem. C. 117(32), 16734–16741 (2013). http://dx.doi.org/10.1021/jp4058109
- Y. He, L. Huang, J.-S. Cai, X.-M. Zheng and S.-G. Sun, “Structure and electrochemical performance of nanostructured Fe3O4/carbon nanotube composites as anodes for lithium ion batteries”, Electrochim. Acta 55(3), 1140–1144 (2010). http://dx.doi.org/10.1016/j.electacta.2009.10.014
- Y. Xue, H. Chen, D. Yu, S. Wang, M. Yardeni, Q. Dai, M. Guo, Y. Liu, F. Lu, J. Qu and L. Dai, “Oxidizing metal ions with graphene oxide: the in situ formation of magnetic nanoparticles on self-reduced graphene sheets for multifunctional applications”, Chem. Commun. 47(42), 11689–11691 (2011). http://dx.doi.org/10.1039/c1cc14789g
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, “Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity”, Pure Appl. Chem. 57(11), 2201–2218 (1982). http://dx.doi.org/10.1351/pac198254112201
- X. Yu, H. Cai, W. Zhang, X. Li, N. Pan, Y. Luo, X. Wang and J. G. Hou, “ Tuning chemical enhancement of SERS by controlling the chemical reduction of graphene oxide nanosheets”, ACS Nano 5(2), 952–958 (2011). http://dx.doi.org/10.1021/nn102291j
- J. N. Tiwari, K. Mahesh, N. H. Le, K. C. Kemp, R. Timilsina, R. N. Tiwari and K. S. Kim, “Reduced graphene oxide-based hydrogels for the efficient capture of dye pollutants from aqueous solutions”, Carbon 56, 173–182 (2013). http://dx.doi.org/10.1016/j.carbon.2013.01.001
- R. Zhang, M. Hummelgård, G. Lv and H. Olin, “Real time monitoring of the drug release of rhodamine B on graphene oxide”, Carbon 49(4), 1126–1132 (2011). http://dx.doi.org/10.1016/j.carbon.2010.11.026
- I. Moreno-Villoslada, M. Jofré, V. Miranda, R. González, T. Sotelo, S. Hess and B. L. Rivas, “pH dependence of the interaction between rhodamine B and the water-soluble poly(sodium 4-styrenesulfonate)”, J. Phys. Chem. B 110(24), 11809–11812 (2006). http://dx.doi.org/10.1021/jp061457j
- A. J. Ahamed, V. Balakrishnan and S. Arivoli, “Kinetic and equilibrium studies of Rhodamine B adsorption by low cost activated carbon”, Arch. Appl. Sci. Res. 3(3), 154–166 (2011).
- G. K. Ramesha, A. V. Kumara, H. B. Muralidhara and S. Sampath, “Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes”, J. Colloid Interf. Sci. 361(1), 270–7 (2011). http://dx.doi.org/10.1016/j.jcis.2011.05.050
- M. Xia, M. Long, Y. Yang, C. Chen, W. Cai and B. Zhou, “A highly active bimetallic oxides catalyst supported on Al-containing MCM-41 for Fenton oxidation of phenol solution”, Appl. Catal. B: Environ. 110, 118–125 (2011). http://dx.doi.org/10.1016/j.apcatb.2011.08.033
- A. Chouket, H. Elhouichet, M. Oueslati, H. Koyama, B. Gelloz and N. Koshida, “Energy transfer in poroussilicon/laser-dye composite evidenced by polarization memory of photoluminescence”, Appl. Phys. Lett. 91(21), 211902 (2007). http://dx.doi.org/10.1063/1.2814051s
References
R. Sanghi and P. Verma, “Decolorisation of aqueous dye solutions by low-cost adsorbents: a review”, Color. Technol. 129(2), 85–108 (2013). http://dx.doi.org/10.1111/cote.12019
A. Dqbrowski, “Adsorption — from theory to practice”, Adv. Colloid Interface Sci. 93(1–3), 135–224 (2001). http://dx.doi.org/10.1016/S0001-8686(00)00082-8
J. C. Lazo-Cannata, A. Nieto-Márquez, A. Jacoby, A. L. Paredes-Doig, A. Romero, M. R. Sun-Kou and J. L. Valverde, “Adsorption of phenol and nitrophenols by carbon nanospheres: Effect of pH and ionic strength”, Sep. Purif. Technol. 80(2), 217–224 (2011). http://dx.doi.org/10.1016/j.seppur.2011.04.029
R. Liu, W. Gong, H. Lan, T. Yang, H. Liu and J. Qu, “Simultaneous removal of arsenate and fluoride by iron and aluminum binary oxide: Competitive adsorption effects”, Sep. Purif. Technol. 92, 100–105 (2012). http://dx.doi.org/10.1016/j.seppur.2012.03.020
X. Hu, B. Liu, Y. Deng, H. Chen, S. Luo, C. Sun, P. Yang and S. Yang, “Adsorption and heterogeneous Fenton degradation of 17α-methyltestosterone on nano Fe3O4/MWCNTs in aqueous solution”, Appl. Catal. B: Environ. 107(3-4), 274–283 (2011). http://dx.doi.org/10.1016/j.apcatb.2011.07.025
S. Tang, N. Lu, J. Li and Y. Wu, “Design and application of an up-scaled dielectric barrier discharge plasma reactor for regeneration of phenolsaturated granular activated carbon”, Sep. Purif. Technol. 95, 73–79 (2012). http://dx.doi.org/10.1016/j.seppur.2012.05.002
V. K. K. Upadhyayula, S. Deng, M. C. Mitchell and G. B. Smith, “Application of carbon nanotube technology for removal of contaminants in drinking water: a review”, Sci. Total Environ. 408(1), 1–13 (2009). http://dx.doi.org/10.1016/j.scitotenv.2009.09.027
Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, “Graphene and graphene oxide: synthesis, properties, and applications”, Adv. Mater. 22(35), 3906–3924 (2010). http://dx.doi.org/10.1002/adma.201001068
J. Kim, L. J. Cote and J. Huang, “Two dimensional soft material: new faces of graphene oxide”, Acc. Chem. Res. 45(8), 1356–1364 (2012). http://dx.doi.org/10.1021/ar300047s
S. Wang, H. Sun, H. M. Ang and M. O. Tadé, “Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials”, Chem. Eng. J. 226, 336–347 (2013). http://dx.doi.org/10.1016/j.cej.2013.04.070
K. C. Kemp, H. Seema, M. Saleh, N. H. Le, K. Mahesh, V. Chandra and K. S. Kim, “Environmental applications using graphene composites: water remediation and gas adsorption”, Nanoscale 5(8), 3149–71 (2013). http://dx.doi.org/10.1039/c3nr33708a
Y. Zhi, G. Rungang, H. Nantao, C. Jing, C. Yingwu, Z. Liying, W. Hao, K. Eric Siu-Wai and Z. Yafei, “The prospective 2D graphene nanosheets: preparation, functionalization and applications”, Nano-Micro Lett. 4(1), 1–9 (2012). http://dx.doi.org/10.3786/nml.v4i1.p1-9
J. Kim, L. J. Cote, F. Kim, W. Yuan, K. R. Shull and J. Huang, “Graphene oxide sheets at interfaces”, J. Am. Chem. Soc. 132(23), 8180–8186 (2010). http://dx.doi.org/10.1021/ja102777p
Z. Liu, J. T. Robinson, X. Sun and H. Dai, “PEGylated nanographene oxide for delivery of water-Insoluble cancer drugs”, J. Am. Chem. Soc. 130(33), 10876–10877 (2008). http://dx.doi.org/10.1021/ja803688x
G. Zhao, T. Wen, C. Chen and X. Wang, “Synthesis of graphene-based nanomaterials and their application in energy-related and environmental-related areas”, RSC Adv. 2(25), 9286–9303 (2012). http://dx.doi.org/10.1039/c2ra20990j
L. Wan, M. Long, D. Zhou, L. Zhang and W. Cai, “Preparation and characterization of freestanding hierarchical porous TiO2 monolith modified with graphene oxide”, Nano-Micro Lett. 4(2), 90–97 (2012). http://dx.doi.org/dx.doi.org/10.3786/nml.v4i2.p90-97
C. T. Yavuz, J. T. Mayo, W. W. Yu, A. Prakash, J. C. Falkner, S. Yean, L. Cong, H. J. Shipley, A. Kan, M. Tomson, D. Natelson and V. L. Colvin, “Low-field magnetic separation of monodisperse Fe3O4 nanocrystals”, Science 314(5801), 964–967 (2006). http://dx.doi.org/10.1126/science.1131475
M. Tayyebeh, A. Abbas, Z. Mohammad Ali, A. Mazaher and K. Nadia, “Application of modified silica coated magnetite nanoparticles for removal of iodine from water samples”, Nano-Micro Lett. 4(1), 57–63 (2012). http://dx.doi.org/10.3786/nml.v4i1.p57-63
H. Sun, L. Cao and L. Lu, “Magnetite/reduced graphene oxide nanocomposites: one step solvothermal synthesis and use as a novel platform for removal of dye pollutants”, Nano Res. 4(6), 550–562 (2011). http://dx.doi.org/10.1007/s12274-011-0111-3
Y. Huang and A. A. Keller, “Magnetic nanoparticle adsorbents for emerging organic contaminants”, ACS Sustainable Chem. Eng. 1(1), 731–736 (2013). http://dx.doi.org/10.1021/sc400047q
L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, “Intrinsic peroxidase-like activity of ferromagnetic nanoparticles”, Nat. Nanotechnol. 2(9), 577–83 (2007). http://dx.doi.org/10.1038/nnano.2007.260
J. Zhang, J. Zhuang, L. Gao, Y. Zhang, N. Gu, J. Feng, D. Yang, J. Zhu and X. Yan, “Decomposing phenol by the hidden talent of ferromagnetic nanoparticles”, Chemosphere 73(9), 1524–1528 (2008). http://dx.doi.org/10.1016/j.chemosphere.2008.05.050
V. Chandra, J. Park, Y. Chun, J. W. Lee, I. C. Hwang and K. S. Kim, “Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal”, ACS Nano 4(7), 3979–3986. (2010). http://dx.doi.org/10.1021/nn1008897
Z. Geng, Y. Lin, X. Yu, Q. Shen, L. Ma, Z. Li, N. Pan and X. Wang, “Highly efficient dye adsorption and removal: a functional hybrid of reduced graphene oxide-Fe3O4 nanoparticles as an easily regenerative adsorbent”, J. Mater. Chem. 22 (8), 3527–3535 (2012). http://dx.doi.org/10.1039/c2jm15544c
F. He, J. Fan, D. Ma, L. Zhang, C. Leung and H. L. Chan, “The attachment of Fe3O4nanoparticles to graphene oxide by covalent bonding”, Carbon 48(11), 3139–3144 (2010). http://dx.doi.org/10.1016/j.carbon.2010.04.052
M. Liu, C. Chen, J. Hu, X. Wu and X. Wang, “Synthesis of magnetite/graphene oxide composite and application for cobalt(II) removal”, J. Phys. Chem. C. 115(51), 25234–25240 (2011). http://dx.doi.org/10.1021/jp208575m
G. Xie, P. Xi, H. Liu, F. Chen, L. Huang, Y. Shi, F. Hou, Z. Zeng, C. Shao and J. Wang, “A facile chemical method to produce superparamagnetic graphene oxide-Fe3O4 hybrid composite and its application in the removal of dyes from aqueous solution”, J. Mater. Chem. 22(3), 1033–1039 (2012). http://dx.doi.org/10.1039/c1jm13433g
X. Zhou, Y. Zhang, C. Wang, X. Wu, Y. Yang, B. Zheng, H. Wu, S. Guo and J. Zhang, “Photo-Fenton reaction of graphene oxide: A new strategy to prepare graphene quantum dots for DNA cleavage”, ACS Nano 6(8), 6592–6599 (2012). http://dx.doi.org/10.1021/nn301629v
S. Q. Liu, B. Xiao, L. R. Feng, S. S. Zhou, Z. G. Chen, C. B. Liu, F. Chen, Z. Y. Wu, N. Xu, W. C. Oh and Z. D. Meng, “Graphene oxide enhances the Fentonlike photocatalytic activity of nickel ferrite for degradation of dyes under visible light irradiation”, Carbon. 64, 197–206 (2013). http://dx.doi.org/10.1016/j.carbon.2013.07.052
Y. C. Lee, S. J. Chang, M. H. Choi, T. J. Jeon, T. Ryu and Y. S. Huh, “Self-assembled graphene oxide with organo-building blocks of Fe-aminoclay for heterogeneous Fenton-like reaction at near-neutral pH: a batch experiment”, Appl. Catal. B-Environ. 142, 494–503 (2013). http://dx.doi.org/10.1016/j.apcatb.2013.05.066
J. J. An, L. H. Zhu, N. Wang, Z. Song, Z. Y. Yang, D. Y. Du and H. Q. Tang, “Photo-Fenton like degradation of tetrabromobisphenol A with graphene-BiFeO3 composite as a catalyst”, Chem. Eng. J. 219, 225–237 (2013). http://dx.doi.org/10.1016/j.cej.2013.01.013
W. S. Hummers and R. E. Offeman, “Preparation of graphitic oxide”, J. Am. Chem. Soc. 80(6), 1339–1339 (1958). http://dx.doi.org/10.1021/ja01539a017
C. Chen, W. Cai, M. Long, B. Zhou, Y. Wu, D. Wu and Y. Feng, “Synthesis of visible light responsive graphene oxide/TiO2 composites with p/n heterojunction”, ACS Nano 4(11), 6425–6432 (2010). http://dx.doi.org/10.1021/nn102130m
G. Wang, X. Shen, B. Wang, J. Yao and J. Park, “Synthesis and characterisation of hydrophilic and organophilic graphene nanosheets.”, Carbon 47(5), 1359–1364 (2009). http://dx.doi.org/10.1016/j.carbon.2009.01.027
Z. J. Fan, W. Kai, J. Yan, T. Wei, L. J. Zhi, J. Feng, Y. Ren, L. P. Song and F. Wei, “Facile synthesis of graphene nanosheets via Fe reduction of exfoliated graphite oxide”, ACS Nano 5(1), 191–198 (2011). http://dx.doi.org/10.1021/nn102339t
P. Wang, J. Wang, T. Ming, X. Wang, H. Yu, J. Yu, Y. Wang and M. Lei, “Dye-sensitization-induced visible-light reduction of graphene oxide for the enhanced TiO2 photocatalytic performance”, ACS Appl. Mater. Interfaces 5(8), 2924–2929 (2013). http://dx.doi.org/10.1021/am4008566
Y. Liu, W. Jiang, Y. Wang, X. J. Zhang, D. Song and F. S. Li, “Synthesis of Fe3O4/CNTs magnetic nanocomposites at the liquid-liquid interface using oleate as surfactant and reactant”, J. Magn. Magn. Mater. 321(5), 408–412 (2009). http://dx.doi.org/10.1016/j.jmmm.2008.09.039
X. Gou, G. Wang, J. Park, H. Liu and J. Yang, “Monodisperse hematite porous nanospheres: synthesis, characterization, and applications for gas sensors”, Nanotechnol. 19(12), 125606 (2008). http://dx.doi.org/10.1088/0957-4484/19/12/125606
O. N. Shebanova and P. Lazor, “Raman study of magnetite (Fe3O4): laser-induced thermal effects and oxidation”, J. Raman Spectrosc. 34(11), 845–852 (2003). http://dx.doi.org/10.1002/jrs.1056
M. Long, Y. Qin, C. Chen, X. Guo, B. Tan and W. Cai, “Origin of visible light photoactivity of RGO/TiO2 by in situ hydrothermal growth of under-grown TiO2 with graphene oxide”, J. Phys. Chem. C. 117(32), 16734–16741 (2013). http://dx.doi.org/10.1021/jp4058109
Y. He, L. Huang, J.-S. Cai, X.-M. Zheng and S.-G. Sun, “Structure and electrochemical performance of nanostructured Fe3O4/carbon nanotube composites as anodes for lithium ion batteries”, Electrochim. Acta 55(3), 1140–1144 (2010). http://dx.doi.org/10.1016/j.electacta.2009.10.014
Y. Xue, H. Chen, D. Yu, S. Wang, M. Yardeni, Q. Dai, M. Guo, Y. Liu, F. Lu, J. Qu and L. Dai, “Oxidizing metal ions with graphene oxide: the in situ formation of magnetic nanoparticles on self-reduced graphene sheets for multifunctional applications”, Chem. Commun. 47(42), 11689–11691 (2011). http://dx.doi.org/10.1039/c1cc14789g
K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, “Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity”, Pure Appl. Chem. 57(11), 2201–2218 (1982). http://dx.doi.org/10.1351/pac198254112201
X. Yu, H. Cai, W. Zhang, X. Li, N. Pan, Y. Luo, X. Wang and J. G. Hou, “ Tuning chemical enhancement of SERS by controlling the chemical reduction of graphene oxide nanosheets”, ACS Nano 5(2), 952–958 (2011). http://dx.doi.org/10.1021/nn102291j
J. N. Tiwari, K. Mahesh, N. H. Le, K. C. Kemp, R. Timilsina, R. N. Tiwari and K. S. Kim, “Reduced graphene oxide-based hydrogels for the efficient capture of dye pollutants from aqueous solutions”, Carbon 56, 173–182 (2013). http://dx.doi.org/10.1016/j.carbon.2013.01.001
R. Zhang, M. Hummelgård, G. Lv and H. Olin, “Real time monitoring of the drug release of rhodamine B on graphene oxide”, Carbon 49(4), 1126–1132 (2011). http://dx.doi.org/10.1016/j.carbon.2010.11.026
I. Moreno-Villoslada, M. Jofré, V. Miranda, R. González, T. Sotelo, S. Hess and B. L. Rivas, “pH dependence of the interaction between rhodamine B and the water-soluble poly(sodium 4-styrenesulfonate)”, J. Phys. Chem. B 110(24), 11809–11812 (2006). http://dx.doi.org/10.1021/jp061457j
A. J. Ahamed, V. Balakrishnan and S. Arivoli, “Kinetic and equilibrium studies of Rhodamine B adsorption by low cost activated carbon”, Arch. Appl. Sci. Res. 3(3), 154–166 (2011).
G. K. Ramesha, A. V. Kumara, H. B. Muralidhara and S. Sampath, “Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes”, J. Colloid Interf. Sci. 361(1), 270–7 (2011). http://dx.doi.org/10.1016/j.jcis.2011.05.050
M. Xia, M. Long, Y. Yang, C. Chen, W. Cai and B. Zhou, “A highly active bimetallic oxides catalyst supported on Al-containing MCM-41 for Fenton oxidation of phenol solution”, Appl. Catal. B: Environ. 110, 118–125 (2011). http://dx.doi.org/10.1016/j.apcatb.2011.08.033
A. Chouket, H. Elhouichet, M. Oueslati, H. Koyama, B. Gelloz and N. Koshida, “Energy transfer in poroussilicon/laser-dye composite evidenced by polarization memory of photoluminescence”, Appl. Phys. Lett. 91(21), 211902 (2007). http://dx.doi.org/10.1063/1.2814051s