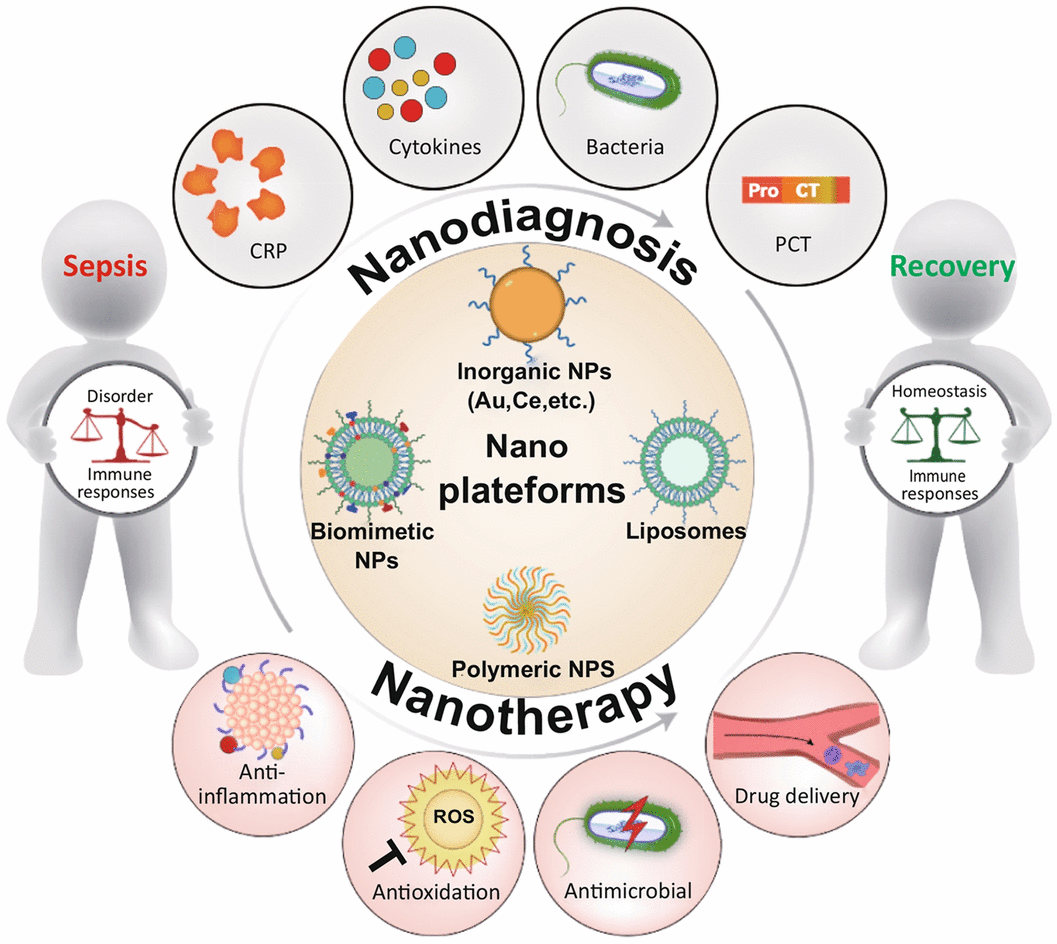
Nanoplatforms for Sepsis Management: Rapid Detection/Warning, Pathogen Elimination and Restoring Immune Homeostasis
Corresponding Author: Xiangming Fang
Nano-Micro Letters,
Vol. 13 (2021), Article Number: 88
Abstract
Sepsis, a highly life-threatening organ dysfunction caused by uncontrollable immune responses to infection, is a leading contributor to mortality in intensive care units. Sepsis-related deaths have been reported to account for 19.7% of all global deaths. However, no effective and specific therapeutic for clinical sepsis management is available due to the complex pathogenesis. Concurrently eliminating infections and restoring immune homeostasis are regarded as the core strategies to manage sepsis. Sophisticated nanoplatforms guided by supramolecular and medicinal chemistry, targeting infection and/or imbalanced immune responses, have emerged as potent tools to combat sepsis by supporting more accurate diagnosis and precision treatment. Nanoplatforms can overcome the barriers faced by clinical strategies, including delayed diagnosis, drug resistance and incapacity to manage immune disorders. Here, we present a comprehensive review highlighting the pathogenetic characteristics of sepsis and future therapeutic concepts, summarizing the progress of these well-designed nanoplatforms in sepsis management and discussing the ongoing challenges and perspectives regarding future potential therapies. Based on these state-of-the-art studies, this review will advance multidisciplinary collaboration and drive clinical translation to remedy sepsis.
Highlights:
1 This review highlights pathogenesis and clinical challenges of sepsis.
2 Advantages of different types of nanoplatforms are presented, and the rationality of nanoplatforms in sepsis management is analyzed.
3 Advances of nanoplatforms in diagnosis and therapy of sepsis are systematically summarized, and ongoing challenges and future perspectives are discussed.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- M. Singer, C.S. Deutschman, C.W. Seymour, M. Shankar-Hari, D. Annane et al., The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315(8), 801–810 (2016). https://doi.org/10.1001/jama.2016.0287
- C. Fleischmann, A. Scherag, N.K. Adhikari, C.S. Hartog, T. Tsaganos et al., International forum of acute care. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Resp. Care 193(3), 259–272 (2016). https://doi.org/10.1164/rccm.201504-0781OC
- D.C. Angus, W.T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo et al., Epidemiology of severe sepsis in the united states: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29(7), 1303–1310 (2001). https://doi.org/10.1097/00003246-00107000-00002
- F.B. Mayr, S. Yende, D.C. Angus, Epidemiology of severe sepsis. Virulence 5(1), 4–11 (2014). https://doi.org/10.4161/viru.27372
- D.J. Funk, J.E. Parrillo, A. Kumar, Sepsis and septic shock: a history. Crit. Care Clin. 25(1), 83–101 (2009). https://doi.org/10.1016/j.ccc.2008.12.003
- SCCM, American college of chest physicians/society of critical care medicine consensus conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 20(6), 864–874 (1992). https://doi.org/10.1097/00003246-199206000-00025
- M.M. Levy, M.P. Fink, J.C. Marshall, E. Abraham, D. Angus et al., 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit. Care Med. 31(4), 1250–1256 (2003). https://doi.org/10.1097/01.CCM.0000050454.01978.3B
- V. Liu, G.J. Escobar, J.D. Greene, J. Soule, A. Whippy et al., Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 312(1), 90–92 (2014). https://doi.org/10.1001/jama.2014.5804
- W.T. Linde-Zwirble, D.C. Angus, Severe sepsis epidemiology: Sampling, selection, and society. Crit. Care 8(4), 222–226 (2004). https://doi.org/10.1186/cc2917
- M. Cecconi, L. Evans, M. Levy, A. Rhodes, Sepsis and septic shock. Lancet 392(10141), 75–87 (2018). https://doi.org/10.1016/s0140-6736(18)30696-2
- B. Cheng, G. Xie, S. Yao, X. Wu, Q. Guo et al., Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in china. Crit. Care Med. 35(11), 2538–2546 (2007). https://doi.org/10.1097/01.CCM.0000284492.30800.00
- K.E. Rudd, S.C. Johnson, K.M. Agesa, K.A. Shackelford, D. Tsoi et al., Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the global burden of disease study. Lancet 395(10219), 200–211 (2020). https://doi.org/10.1016/s0140-6736(19)32989-7
- T. van der Poll, F.L. van de Veerdonk, B.P. Scicluna, M.G. Netea, The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 17(7), 407–420 (2017). https://doi.org/10.1038/nri.2017.36
- M.P. Fink, H.S. Warren, Strategies to improve drug development for sepsis. Nat. Rev. Drug Discov. 13(10), 741–758 (2014). https://doi.org/10.1038/nrd4368
- K.N. Iskander, M.F. Osuchowski, D.J. Stearns-Kurosawa, S. Kurosawa, D. Stepien et al., Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Phys. Rev. 93(3), 1247–1288 (2013). https://doi.org/10.1152/physrev.00037.2012
- R.S. Hotchkiss, L.L. Moldawer, S.M. Opal, K. Reinhart, I.R. Turnbull et al., Sepsis and septic shock. Nat. Rev. Dis. Primers 2(1), 16045 (2016). https://doi.org/10.1038/nrdp.2016.45
- F. Venet, G. Monneret, Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 14(2), 121–137 (2018). https://doi.org/10.1038/nrneph.2017.165
- J.C. Marshall, Why have clinical trials in sepsis failed? Trends Mol. Med. 20(4), 195–203 (2014). https://doi.org/10.1016/j.molmed.2014.01.007
- J.L. Halbach, A.W. Wang, D. Hawisher, D.M. Cauvi, R.E. Lizardo et al., Why antibiotic treatment is not enough for sepsis resolution: an evaluation in an experimental animal model. Infect. Immun. 85(12), e0066400617 (2017). https://doi.org/10.1128/IAI.00664-17
- J.C. Hou, Q. Chen, K. Zhang, B.L. Cheng, G.H. Xie et al., Sphingosine 1-phosphate receptor 2 signaling suppresses macrophage phagocytosis and impairs host defense against sepsis. Anesthesiology 123(2), 409–422 (2015). https://doi.org/10.1097/ALN.0000000000000725
- F. Song, J. Hou, Z. Chen, B. Cheng, R. Lei et al., Sphingosine-1-phosphate receptor 2 signaling promotes caspase-11-dependent macrophage pyroptosis and worsens escherichia coli sepsis outcome. Anesthesiology 129(2), 311–320 (2018). https://doi.org/10.1097/ALN.0000000000002196
- F. Niessen, F. Schaffner, C. Furlan-Freguia, R. Pawlinski, G. Bhattacharjee et al., Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature 452(7187), 654–658 (2008). https://doi.org/10.1038/nature06663
- J. Hou, Q. Chen, X. Wu, D. Zhao, H. Reuveni et al., S1PR3 signaling drives bacterial killing and is required for survival in bacterial sepsis. Am. J. Resp. Crit Care 196(12), 1559–1570 (2017). https://doi.org/10.1164/rccm.201701-0241OC
- A. Bouchon, F. Facchetti, M.A. Weigand, M. Colonna, Trem-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410(6832), 1103–1107 (2001). https://doi.org/10.1038/35074114
- Q. Chen, K. Zhang, Y. Jin, T. Zhu, B. Cheng et al., Triggering receptor expressed on myeloid cells-2 protects against polymicrobial sepsis by enhancing bacterial clearance. Am. J. Resp. Crit. Care 188(2), 201–212 (2013). https://doi.org/10.1164/rccm.201211-1967OC
- Q. Chen, Y. Jin, K. Zhang, H. Li, W. Chen et al., Alarmin HNP-1 promotes pyroptosis and IL-1beta release through different roles of NLRP3 inflammasome via P2X7 in LPS-primed macrophages. Innate Immun. 20(3), 290–300 (2014). https://doi.org/10.1177/1753425913490575
- Q. Chen, Y. Yang, J. Hou, Q. Shu, Y. Yin et al., Increased gene copy number of defa1/defa3 worsens sepsis by inducing endothelial pyroptosis. Proc. Natl. Acad. Sci. USA 116(8), 3161–3170 (2019). https://doi.org/10.1073/pnas.1812947116
- N. Arulkumaran, M.L. Sixma, S. Pollen, E. Ceravola, E. Jentho et al., P2X7 receptor antagonism ameliorates renal dysfunction in a rat model of sepsis. Phys. Rep. 6(5), e13622 (2018). https://doi.org/10.14814/phy2.13622
- X.W. Qian, T. Numata, K. Zhang, C.X. Li, J.C. Hou et al., Transient receptor potential melastatin 2 protects mice against polymicrobial sepsis by enhancing bacterial clearance. Anesthesiology 121(2), 336–351 (2014). https://doi.org/10.1097/ALN.0000000000000275
- Z. Zhang, P. Cui, K. Zhang, Q. Chen, X. Fang, Transient receptor potential melastatin 2 regulates phagosome maturation and is required for bacterial clearance in escherichia coli sepsis. Anesthesiology 126(1), 128–139 (2017). https://doi.org/10.1097/ALN.0000000000001430
- L. Wang, T.-M. Fu, Y. Zhou, S. Xia, A. Greka et al., Structures and gating mechanism of human TRPM2. Science 362(eaav6421), 4809 (2018). https://doi.org/10.1126/science.aav4809
- H.R. Schmidt, S. Zheng, E. Gurpinar, A. Koehl, A. Manglik et al., Crystal structure of the human sigma1 receptor. Nature 532(7600), 527–530 (2016). https://doi.org/10.1038/nature17391
- D.A. Rosen, S.M. Seki, A. Fernández-Castañeda, R.M. Beiter, J.D. Eccles et al., Modulation of the sigma-1 receptor–IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci. Transl. Med. 11, eaau5266 (2019). https://doi.org/10.1126/scitranslmed.aau5266
- A. Rhodes, L.E. Evans, W. Alhazzani, M.M. Levy, M. Antonelli et al., Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intens. Care Med. 43(3), 304–377 (2017). https://doi.org/10.1007/s00134-017-4683-6
- C.W. Seymour, F. Gesten, H.C. Prescott, M.E. Friedrich, T.J. Iwashyna et al., Time to treatment and mortality during mandated emergency care for sepsis. N. Engl. J. Med. 376(23), 2235–2244 (2017). https://doi.org/10.1056/NEJMoa1703058
- B.-T. Huynh, M. Padget, B. Garin, E. Delarocque-Astagneau, D. Guillemot, Bacterial neonatal sepsis and antibiotic resistance in low-income countries. Lancet 387(10018), 533–534 (2016). https://doi.org/10.1016/s0140-6736(16)00220-8
- K.E. Drexler, Molecular engineering: an approach to the development of general capabilities for molecular manipulation. Proc. Natl. Acad. Sci. USA 78(9), 5275–5278 (1981). https://doi.org/10.1073/pnas.78.9.5275
- R.T. Sadikot, The potential role of nano- and micro-technology in the management of critical illnesses. Adv. Drug Deliver. Rev. 77(1), 27–31 (2014). https://doi.org/10.1016/j.addr.2014.07.004
- J. Shi, P.W. Kantoff, R. Wooster, O.C. Farokhzad, Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 17(1), 20–37 (2017). https://doi.org/10.1038/nrc.2016.108
- W. Jiang, H. Yuan, C.K. Chan, C.A. von Roemeling, Z. Yan et al., Lessons from immuno-oncology: a new era for cancer nanomedicine? Nat. Rev. Drug Discov. 16(6), 369–370 (2017). https://doi.org/10.1038/nrd.2017.34
- M.E. Lobatto, V. Fuster, Z.A. Fayad, W.J. Mulder, Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 10(11), 835–852 (2011). https://doi.org/10.1038/nrd3578
- O. Veiseh, B.C. Tang, K.A. Whitehead, D.G. Anderson, R. Langer, Managing diabetes with nanomedicine: challenges and opportunities. Nat. Rev. Drug Discov. 14(1), 45–57 (2015). https://doi.org/10.1038/nrd4477
- A.D. Smith, Big moment for nanotech: oncology therapeutics poised for a leap (2013)
- G. Luo, Q. Yang, B. Yao, Y. Tian, R. Hou et al., SLP-coated liposomes for drug delivery and biomedical applications: potential and challenges. Int. J. Nanomed. 14(1), 1359–1383 (2019). https://doi.org/10.2147/IJN.S189935
- W. Wang, A. Shao, S. Feng, M. Ding, G. Luo, Physicochemical characterization and gastrointestinal adhesion of s-layer proteins-coating liposomes. Int. J. Pharmaceut. 529(1–2), 227–237 (2017). https://doi.org/10.1016/j.ijpharm.2017.07.006
- A. Bernkop-Schnurch, A. Jalil, Do drug release studies from sedds make any sense? J. Control. Release 271(1), 55–59 (2018). https://doi.org/10.1016/j.jconrel.2017.12.027
- C. Kinnear, T.L. Moore, L. Rodriguez-Lorenzo, B. Rothen-Rutishauser, A. Petri-Fink, Form follows function: nanoparticle shape and its implications for nanomedicine. Chem. Rev. 117(17), 11476–11521 (2017). https://doi.org/10.1021/acs.chemrev.7b00194
- H. Wang, H. Xie, J. Wang, J. Wu, X. Ma et al., Self-assembling prodrugs by precise programming of molecular structures that contribute distinct stability, pharmacokinetics, and antitumor efficacy. Adv. Funct. Mater. 25(31), 4956–4965 (2015). https://doi.org/10.1002/adfm.201501953
- J. Wan, Y. Qiao, X. Chen, J. Wu, L. Zhou et al., Structure-guided engineering of cytotoxic cabazitaxel for an adaptive nanoparticle formulation: Enhancing the drug safety and therapeutic efficacy. Adv. Funct. Mater. 28(52), 1804229 (2018). https://doi.org/10.1002/adfm.201804229
- S.A. Anuj, H.P. Gajera, D.G. Hirpara, B.A. Golakiya, Bactericidal assessment of nano-silver on emerging and re-emerging human pathogens. J. Trace Elem. Med. Biol. 51, 219–225 (2019). https://doi.org/10.1016/j.jtemb.2018.04.028
- Y. Li, Y. Chang, X. Lian, L. Zhou, Z. Yu et al., Silver nanoparticles for enhanced cancer theranostics: in vitro and in vivo perspectives. J. Biomed. Nanotechnol. 14(9), 1515–1542 (2018). https://doi.org/10.1166/jbn.2018.2614
- J.R. Melamed, R.S. Riley, D.M. Valcourt, E.S. Day, Using gold nanoparticles to disrupt the tumor microenvironment: an emerging therapeutic strategy. ACS Nano 10(12), 10631–10635 (2016). https://doi.org/10.1021/acsnano.6b07673
- W. Gao, Y. Xiong, Q. Li, H. Yang, Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front. Physiol. 8(1), 508 (2017). https://doi.org/10.3389/fphys.2017.00508
- P. Cui, X. Fang, Pathogenesis of infection in surgical patients. Curr. Opin. Crit. Care 21(4), 343–350 (2015). https://doi.org/10.1097/MCC.0000000000000227
- R. Lei, J. Hou, Q. Chen, W. Yuan, B. Cheng et al., Self-assembling myristoylated human alpha-defensin 5 as a next-generation nanobiotics potentiates therapeutic efficacy in bacterial infection. ACS Nano 12, 5284–5296 (2018). https://doi.org/10.1021/acsnano.7b09109
- A. Balakrishnan, P. DasSarma, O. Bhattacharjee, J.M. Kim, S. DasSarma et al., Halobacterial nano vesicles displaying murine bactericidal permeability-increasing protein rescue mice from lethal endotoxic shock. Sci. Rep. 6, 33679 (2016). https://doi.org/10.1038/srep33679
- F.H. Liao, T.H. Wu, Y.T. Huang, W.J. Lin, C.J. Su et al., Subnanometer gold clusters adhere to lipid a for protection against endotoxin-induced sepsis. Nano Lett. 18(5), 2864–2869 (2018). https://doi.org/10.1021/acs.nanolett.7b05464
- J.L. Perry, K.P. Herlihy, M. Napier, J.M. Desimon, Print: a novel platform toward shape and size specific nanoparticle theranostics. Acc. Chem. Res. 44(10), 990–998 (2011). https://doi.org/10.1021/ar2000315
- S. Mura, P. Couvreur, Nanotheranostics for personalized medicine. Adv. Drug Deliver. Rev. 64(13), 1394–1416 (2012). https://doi.org/10.1016/j.addr.2012.06.006
- D. Peer, J.M. Karp, S. Hong, O.C. Farokhzad, R. Margalit et al., Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760 (2007). https://doi.org/10.1038/nnano.2007.387
- B.R. Smith, S.S. Gambhir, Nanomaterials for in vivo imaging. Chem. Rev. 117(3), 901–986 (2017). https://doi.org/10.1021/acs.chemrev.6b00073
- F.M. Kievit, M. Zhang, Cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers. Adv. Mater. 23(36), H217-247 (2011). https://doi.org/10.1002/adma.201102313
- W.L. Tang, W.H. Tang, S.D. Li, Cancer theranostic applications of lipid-based nanoparticles. Drug Discov. Today 23(5), 1159–1166 (2018). https://doi.org/10.1016/j.drudis.2018.04.007
- Y. Liu, P. Bhattarai, Z. Dai, X. Chen, Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 48(7), 2053–2108 (2019). https://doi.org/10.1039/c8cs00618k
- S. Mohamed, S. Veeranarayanan, T. Maekawa, S. Kumar, External stimulus responsive inorganic nanomaterials for cancer theranostics. Adv. Drug Deliver. Rev. 138(1), 18–40 (2019). https://doi.org/10.1016/j.addr.2018.10.007
- P. Jagtap, V. Sritharan, S. Gupta, Nanotheranostic approaches for management of bloodstream bacterial infections. Nanomed-Nanotechnol. 13(1), 329–341 (2017). https://doi.org/10.1016/j.nano.2016.09.005
- M.D. Howard, E.D. Hood, B. Zern, V.V. Shuvaev, T. Grosser et al., Nanocarriers for vascular delivery of anti-inflammatory agents. Annu. Rev. Pharmacol. 54(1), 205–226 (2014). https://doi.org/10.1146/annurev-pharmtox-011613-140002
- A. Kumar, R.K. Chaudhary, R. Singh, S.P. Singh, S.Y. Wang et al., Nanotheranostic applications for detection and targeting neurodegenerative diseases. Front. Neurosci. 14(1), 305 (2020). https://doi.org/10.3389/fnins.2020.00305
- F. Liu, L. Lin, Y. Zhang, Y. Wang, S. Sheng et al., A tumor-microenvironment-activated nanozyme-mediated theranostic nanoreactor for imaging-guided combined tumor therapy. Adv. Mater. 31(40), e1902885 (2019). https://doi.org/10.1002/adma.201902885
- C.F. Markwalter, A.G. Kantor, C.P. Moore, K.A. Richardson, D.W. Wright, Inorganic complexes and metal-based nanomaterials for infectious disease diagnostics. Chem. Rev. 119(2), 1456–1518 (2019). https://doi.org/10.1021/acs.chemrev.8b00136
- B. Yang, Y. Chen, J. Shi, Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 119(8), 4881–4985 (2019). https://doi.org/10.1021/acs.chemrev.8b00626
- R. Eivazzadeh-Keihan, E. Bahojb-Noruzi, K. Khanmohammadi-Chenab, A. Jafari, F. Radinekiyan et al., Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 14, 1687–1714 (2020). https://doi.org/10.1002/term.3131
- G. Yang, S.Z.F. Phua, A.K. Bindra, Y. Zhao, Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv. Mater. 31(10), e1805730 (2019). https://doi.org/10.1002/adma.201805730
- H.P. Lee, A.K. Gaharwar, Light-responsive inorganic biomaterials for biomedical applications. Adv. Sci. 7(17), 2000863 (2020). https://doi.org/10.1002/advs.202000863
- J.H. Lee, H.Y. Cho, H.K. Choi, J.Y. Lee, J.W. Choi, Application of gold nanoparticle to plasmonic biosensors. Int. J. Mol. Sci. 19(7), 2021 (2018). https://doi.org/10.3390/ijms19072021
- N. Wang, H. Dai, L. Sai, H. Ma, M. Lin, Copper ion-assisted gold nanoparticle aggregates for electrochemical signal amplification of lipopolysaccharide sensing. Biosens. Bioelectron. 126, 529–534 (2019). https://doi.org/10.1016/j.bios.2018.11.021
- J.H. Kim, J.S. Suh, J. Yang, Labeling-free detection of ECD-HER2 protein using aptamer-based nano-plasmonic sensor. Nanotechnology 31(17), 175501 (2020). https://doi.org/10.1088/1361-6528/ab68fa
- V.A. Tran, V.G. Vo, K. Shim, S.W. Lee, S.S.A. An, Multimodal mesoporous silica nanocarriers for dual stimuli-responsive drug release and excellent photothermal ablation of cancer cells. Int. J. Nanomed. 15(1), 7667–7685 (2020). https://doi.org/10.2147/IJN.S254344
- Y. Zhang, C. Guo, L. Liu, J. Xu, H. Jiang et al., Zno-based multifunctional nanocomposites to inhibit progression and metastasis of melanoma by eliciting antitumor immunity via immunogenic cell death. Theranostics 10(24), 11197–11214 (2020). https://doi.org/10.7150/thno.44920
- D. Jiang, D. Ni, Z.T. Rosenkrans, P. Huang, X. Yan et al., Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 48(14), 3683–3704 (2019). https://doi.org/10.1039/c8cs00718g
- S. Gao, H. Lin, H. Zhang, H. Yao, Y. Chen et al., Nanocatalytic tumor therapy by biomimetic dual inorganic nanozyme-catalyzed cascade reaction. Adv. Sci. 6(3), 1801733 (2019). https://doi.org/10.1002/advs.201801733
- X. Hu, F. Li, F. Xia, X. Guo, N. Wang et al., Biodegradation-mediated enzymatic activity-tunable molybdenum oxide nanourchins for tumor-specific cascade catalytic therapy. J. Am. Chem. Soc. 142(3), 1636–1644 (2020). https://doi.org/10.1021/jacs.9b13586
- Y.F. Liu, Y. Cheng, H. Zhang, M. Zhou, Y.J. Yu et al., Integrated cascade nanozyme catalyzes in vivo ROS scavenging for anti-inflammatory therapy. Sci. Adv. 6(1), 2695 (2020). https://doi.org/10.1126/sciadv.abb2695
- N. Kamaly, B. Yameen, J. Wu, O.C. Farokhzad, Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem. Rev. 116(4), 2602–2663 (2016). https://doi.org/10.1021/acs.chemrev.5b00346
- K. Ulbrich, K. Hola, V. Subr, A. Bakandritsos, J. Tucek et al., Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 116(9), 5338–5431 (2016). https://doi.org/10.1021/acs.chemrev.5b00589
- I. Ekladious, Y.L. Colson, M.W. Grinstaff, Polymer-drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 18(4), 273–294 (2019). https://doi.org/10.1038/s41573-018-0005-0
- S. Rashki, K. Asgarpour, H. Tarrahimofrad, M. Hashemipour, M.S. Ebrahimi et al., Chitosan-based nanoparticles against bacterial infections. Carbohyd. Polym. 251(1), 117108 (2021). https://doi.org/10.1016/j.carbpol.2020.117108
- M. Lara-Velazquez, R. Alkharboosh, E.S. Norton, C. Ramirez-Loera, W.D. Freeman et al., Chitosan-based non-viral gene and drug delivery systems for brain cancer. Front. Neurol. 11(1), 740 (2020). https://doi.org/10.3389/fneur.2020.00740
- X. Lang, T. Wang, M. Sun, X. Chen, Y. Liu, Advances and applications of chitosan-based nanomaterials as oral delivery carriers: a review. Int. J. Biol. Macromol. 154(1), 433–445 (2020). https://doi.org/10.1016/j.ijbiomac.2020.03.148
- P. Severino, C.F. da Silva, L.N. Andrade, D. de Lima-Oliveira, J. Campos et al., Alginate nanoparticles for drug delivery and targeting. Curr. Pharm. Des. 25(11), 1312–1334 (2019). https://doi.org/10.2174/1381612825666190425163424
- J. Ding, Y. Yao, J. Li, Y. Duan, J.R. Nakkala et al., A reactive oxygen species scavenging and O2 generating injectable hydrogel for myocardial infarction treatment in vivo. Small 16, 2005038 (2020). https://doi.org/10.1002/smll.202005038
- Y. Hong, F. Zhou, Y. Hua, X. Zhang, C. Ni et al., A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 10(1), 2060 (2019). https://doi.org/10.1038/s41467-019-10004-7
- Y.W. Chen, W.L. Shen, C.Q. Tang, J.Y. Huang, C.M. Fan et al., Targeted pathological collagen delivery of sustained-release rapamycin to prevent heterotopic ossification. Sci. Adv. 6(1), 9526 (2019). https://doi.org/10.1126/sciadv.aay9526
- J. Di, F. Xie, Y. Xu, When liposomes met antibodies: drug delivery and beyond. Adv. Drug Deliver. Rev. (2020). https://doi.org/10.1016/j.addr.2020.09.003
- J.J. Sonju, A. Dahal, S.S. Singh, S.D. Jois, Peptide-functionalized liposomes as therapeutic and diagnostic tools for cancer treatment. J. Control. Release (2020). https://doi.org/10.1016/j.jconrel.2020.09.055
- A. Al-Saqr, M.F. Aldawsari, H. Alrbyawi, I. Poudel, M. Annaji et al., Co-delivery of hispolon and doxorubicin liposomes improves efficacy against melanoma cells. AAPS PharmSciTech 21(8), 304 (2020). https://doi.org/10.1208/s12249-020-01846-2
- Q. Wang, P. Tardi, N. Sadowski, S. Xie, D. Heller et al., Pharmacokinetics, drug metabolism, and tissue distribution of CPX-351 in animals. Nanomed. Nanotechnol. 30(1), 102275 (2020). https://doi.org/10.1016/j.nano.2020.102275
- L. Shi, Y. Wang, Q. Wang, Z. Jiang, L. Ren et al., Transforming a toxic drug into an efficacious nanomedicine using a lipoprodrug strategy for the treatment of patient-derived melanoma xenografts. J. Control. Release 324(1), 289–302 (2020). https://doi.org/10.1016/j.jconrel.2020.05.025
- Y. Tang, X. Wang, J. Li, Y. Nie, G. Liao et al., Overcoming the reticuloendothelial system barrier to drug delivery with a “don’t-eat-us” strategy. ACS Nano 13(11), 13015–13026 (2019). https://doi.org/10.1021/acsnano.9b05679
- Z. Zhang, J. Guan, Z. Jiang, Y. Yang, J. Liu et al., Brain-targeted drug delivery by manipulating protein corona functions. Nat. Commun. 10(1), 3561 (2019). https://doi.org/10.1038/s41467-019-11593-z
- H. Wang, X. Xu, X. Guan, S. Shen, X. Huang et al., Liposomal 9-aminoacridine for treatment of ischemic stroke: from drug discovery to drug delivery. Nano Lett. 20(3), 1542–1551 (2020). https://doi.org/10.1021/acs.nanolett.9b04018
- J. Zhou, A.V. Kroll, M. Holay, R.H. Fang, L. Zhang, Biomimetic nanotechnology toward personalized vaccines. Adv. Mater. 32(13), e1901255 (2020). https://doi.org/10.1002/adma.201901255
- Z. Chen, Z. Wang, Z. Gu, Bioinspired and biomimetic nanomedicines. Acc. Chem. Res. 52(5), 1255–1264 (2019). https://doi.org/10.1021/acs.accounts.9b00079
- R.H. Fang, A.V. Kroll, W. Gao, L. Zhang, Cell membrane coating nanotechnology. Adv. Mater. 30(23), e1706759 (2018). https://doi.org/10.1002/adma.201706759
- J.C. Harris, M.A. Scully, E.S. Day, Cancer cell membrane-coated nanoparticles for cancer management. Cancers 11(12), 1836 (2019). https://doi.org/10.3390/cancers11121836
- V. Cagno, P. Andreozzi, M. D’Alicarnasso, P. Jacob-Silva, M. Mueller et al., Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 17(2), 195–203 (2018). https://doi.org/10.1038/nmat5053
- J. Wang, P. Li, Y. Yu, Y. Fu, H. Jiang et al., Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 367(6480), 0810 (2020). https://doi.org/10.1126/science.aau0810
- Y. Liu, J. Luo, X. Chen, W. Liu, T. Chen, Cell membrane coating technology: a promising strategy for biomedical applications. Nano-Micro Lett. 11(1), 100 (2019). https://doi.org/10.1007/s40820-019-0330-9
- R. Molinaro, C. Corbo, J.O. Martinez, F. Taraballi, M. Evangelopoulos et al., Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 15(9), 1037–1046 (2016). https://doi.org/10.1038/nmat4644
- J. Koo, T. Escajadillo, L. Zhang, V. Nizet, S.M. Lawrence, Erythrocyte-coated nanoparticles block cytotoxic effects of group b streptococcus beta-hemolysin/cytolysin. Front. Pediatr. 7(1), 410 (2019). https://doi.org/10.3389/fped.2019.00410
- Y. Lu, Q. Hu, C. Jiang, Z. Gu, Platelet for drug delivery. Curr. Opin. Biotechnol. 58(1), 81–91 (2019). https://doi.org/10.1016/j.copbio.2018.11.010
- J. Zhuang, H. Gong, J.R. Zhou, Q.Z. Zhang, W.W. Gao et al., Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles. Sci. Adv. 6(1), 6108 (2020). https://doi.org/10.1126/sciadv.aaz6108
- G. Zhang, G.R. Campbell, Q.Z. Zhang, E. Maule, J. Hanna et al., Cd4+ t cell-mimicking nanoparticles broadly neutralize HIV-1 and suppress viral replication through autophagy. mBio 11(5), 00903–00920 (2020). https://doi.org/10.1128/mBio.00903-20
- C. Tapeinos, F. Tomatis, M. Battaglini, A. Larranaga, A. Marino et al., Cell membrane-coated magnetic nanocubes with a homotypic targeting ability increase intracellular temperature due to ros scavenging and act as a versatile theranostic system for glioblastoma multiforme. Adv. Health Mater. 8(18), e1900612 (2019). https://doi.org/10.1002/adhm.201900612
- Y. Jiang, N. Krishnan, J. Zhou, S. Chekuri, X. Wei et al., Engineered cell-membrane-coated nanoparticles directly present tumor antigens to promote anticancer immunity. Adv. Mater. 32(30), e2001808 (2020). https://doi.org/10.1002/adma.202001808
- Y. Zhang, Y. Chen, C. Lo, J. Zhuang, P. Angsantikul et al., Inhibition of pathogen adhesion by bacterial outer membrane-coated nanoparticles. Angew. Chem. Int. Ed. 58(33), 11404–11408 (2019). https://doi.org/10.1002/anie.201906280
- Q. Zhang, A. Honko, J. Zhou, H. Gong, S.N. Downs et al., Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett. 20(7), 5570–5574 (2020). https://doi.org/10.1021/acs.nanolett.0c02278
- F. Gorjikhah, S. Davaran, R. Salehi, M. Bakhtiari, A. Hasanzadeh et al., Improving “lab-on-a-chip” techniques using biomedical nanotechnology: a review. Artif. Cell. Nanomed. Biotechnol. 44(7), 1609–1614 (2016). https://doi.org/10.3109/21691401.2015.1129619
- B. Hu, C. Owh, P.L. Chee, W.R. Leow, X. Liu et al., Supramolecular hydrogels for antimicrobial therapy. Chem. Soc. Rev. 47(18), 6917–6929 (2018). https://doi.org/10.1039/c8cs00128f
- S. Wang, Y. Gao, Q. Jin, J. Ji, Emerging antibacterial nanomedicine for enhanced antibiotic therapy. Biomater. Sci. 8, 6825–6839 (2020). https://doi.org/10.1039/d0bm00974a
- D.G. Remick, Cytokine therapeutics for the treatment of sepsis: why has nothing worked? Curr. Pharm. Des. 9(1), 75–82 (2003). https://doi.org/10.2174/1381612033392567
- M.D.V. Marco-Ranieri, M.D.B. Taylor-Thompson, M.D. Philip, S. Barie, M.D. Jean-François-Dhainaut et al., Drotrecogin alfa (activated) in adults with septic shock. N. Engl. J. Med. 366(22), 2055–2064 (2012). https://doi.org/10.1056/NEJMoa1202290
- J. Lou-Franco, B. Das, C. Elliott, C. Cao, Gold nanozymes: from concept to biomedical applications. Nano-Micro Lett. 13(1), 10 (2020). https://doi.org/10.1007/s40820-020-00532-z
- N. Feliu, D. Docter, M. Heine, P. Del Pino, S. Ashraf et al., In vivo degeneration and the fate of inorganic nanoparticles. Chem. Soc. Rev. 45(9), 2440–2457 (2016). https://doi.org/10.1039/c5cs00699f
- N.D. Donahue, H. Acar, S. Wilhelm, Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliver. Rev. 143(1), 68–96 (2019). https://doi.org/10.1016/j.addr.2019.04.008
- S. Ravindran, J.K. Suthar, R. Rokade, P. Deshpande, P. Singh et al., Pharmacokinetics, metabolism, distribution and permeability of nanomedicine. Curr. Drug Metab. 19(4), 327–334 (2018). https://doi.org/10.2174/1389200219666180305154119
- S.E. McNeil, Nanoparticle therapeutics a personal perspective. Wires Nanomed. Nanobi. 1(1), 264–271 (2009). https://doi.org/10.1002/wnan.006
- S. Ranjbar-Bahadori, A. Mulgaonkar, R. Hart, C.Y. Wu, D. Zhang et al., Radiolabeling strategies and pharmacokinetic studies for metal based nanotheranostics. Wires Nanomed. Nanobi. (2020). https://doi.org/10.1002/wnan.1671
- H. Arami, A. Khandhar, D. Liggitt, K.M. Krishnan, In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 44(23), 8576–8607 (2015). https://doi.org/10.1039/c5cs00541h
- J. Lu, J. Wang, D. Ling, Surface engineering of nanoparticles for targeted delivery to hepatocellular carcinoma. Small 14(5), 1702037 (2018). https://doi.org/10.1002/smll.201702037
- E. Ben-Akiva, R.A. Meyer, H.Z. Yu, J.T. Smith, D.M. Pardoll et al., Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Sci. Adv. 6(1), 9035 (2020). https://doi.org/10.1126/sciadv.aay9035
- F. Bloos, K. Reinhart, Rapid diagnosis of sepsis. Virulence 5(1), 154–160 (2014). https://doi.org/10.4161/viru.27393
- X.Z. Mou, X.Y. Chen, J. Wang, Z. Zhang, Y. Yang et al., Bacteria-instructed click chemistry between functionalized gold nanoparticles for point-of-care microbial detection. ACS Appl. Mater. Interfaces 11(26), 23093–23101 (2019). https://doi.org/10.1021/acsami.9b09279
- M. Sun, A. Qu, C. Hao, X. Wu, L. Xu et al., Chiral upconversion heterodimers for quantitative analysis and bioimaging of antibiotic-resistant bacteria in vivo. Adv. Mater. 30(50), e1804241 (2018). https://doi.org/10.1002/adma.201804241
- I.K. Herrmann, S. Bertazzo, D.J.P. O’Callaghan, A.A. Schlegel et al., Differentiating sepsis from non-infectious systemic inflammation based on microvesicle-bacteria aggregation. Nanoscale 7(32), 13511–13520 (2015). https://doi.org/10.1039/c5nr01851j
- L. Wang, W.J. Zhao, M.B. O’Donoghue, W. Tan, Fluorescent nanoparticles for multiplexed bacteria monitoring. Bioconjugate Chem. 18(2), 297–301 (2007). https://doi.org/10.1021/bc060255n
- G. Santopolo, A. Doménech-Sánchez, S.M. Russell, R. de la Rica, Ultrafast and ultrasensitive naked-eye detection of urease-positive bacteria with plasmonic nanosensors. ACS Sensors 4(4), 961–967 (2019). https://doi.org/10.1021/acssensors.9b00063
- C.-W. Lee, H.-Y. Chang, J.-K. Wu, F.-G. Tseng, Ultra-sensitive electrochemical detection of bacteremia enabled by redox-active gold nanoparticles (raGNPs) in a nano-sieving microfluidic system (NS-MFS). Biosens. Bioelectron. 133(1), 215–222 (2019). https://doi.org/10.1016/j.bios.2019.03.040
- J. Gao, L. Li, P.L. Ho, G.C. Mak, H. Gu et al., Combining fluorescent probes and biofunctional magnetic nanoparticles for rapid detection of bacteria in human blood. Adv. Mater. 18(23), 3145–3148 (2006). https://doi.org/10.1002/adma.200601058
- A. Belushkin, F. Yesilkoy, H. Altug, Nanoparticle-enhanced plasmonic biosensor for digital biomarker detection in a microarray. ACS Nano 12(5), 4453–4461 (2018). https://doi.org/10.1021/acsnano.8b00519
- D.K. Changlin-Fu, Pilot application of magnetic nanoparticle-based biosensor for necrotizing enterocolitis. J. Proteomics. Bioinf. 5(1), 002 (2015). https://doi.org/10.4172/jpb.S5-002
- R.K. Gupta, A. Periyakaruppan, M. Meyyappan, J.E. Koehne, Label-free detection of c-reactive protein using a carbon nanofiber based biosensor. Biosens. Bioelectron. 59(1), 112–119 (2014). https://doi.org/10.1016/j.bios.2014.03.027
- L. Cao, J. Kiely, M. Piano, R. Luxton, A copper oxide/zinc oxide composite nano-surface for use in a biosensor. Materials 12(7), 1126 (2019). https://doi.org/10.3390/ma12071126
- L. Zhang, S. Tong, J. Zhou, G. Bao, Accurate quantification of disease markers in human serum using iron oxide nanoparticle-linked immunosorbent assay. Theranostics 6(9), 1353–1361 (2016). https://doi.org/10.7150/thno.16093
- L. Cao, J. Kiely, M. Piano, R. Luxton, Facile and inexpensive fabrication of zinc oxide based bio-surfaces for c-reactive protein detection. Sci. Rep. 8(1), 12687 (2018). https://doi.org/10.1038/s41598-018-30793-z
- K.M. Park, D.J. Chung, M. Choi, T. Kang, J. Jeong, Fluorescent fullerene nanoparticle-based lateral flow immunochromatographic assay for rapid quantitative detection of c-reactive protein. Nano Converg. 6(1), 35 (2019). https://doi.org/10.1186/s40580-019-0207-0
- I. Letchumanan, M.K. Md-Arshad, S.R. Balakrishnan, S.C.B. Gopinath, Gold-nanorod enhances dielectric voltammetry detection of c-reactive protein: a predictive strategy for cardiac failure. Biosens. Bioelectron. 130(1), 40–47 (2019). https://doi.org/10.1016/j.bios.2019.01.042
- A. Prajapati, N. Verma, A. Pandya, Highly sensitive vertical flow based point-of-care immunokit for rapid and early detection of human CRP as a cardiovascular risk factor. Biomed. Microdevices 22(2), 28 (2020). https://doi.org/10.1007/s10544-020-00480-w
- A. Liu, X. Wang, Amperometric immunosensor of procalcitonin based on amplification strategy of ferrocene-modified gold nanoparticles. Int. J. Electrochem. Sci. 10(1), 9342–9350 (2015)
- P. Li, W. Zhang, X. Zhou, L. Zhang, C60 carboxyfullerene-based functionalised nanohybrids as signal-amplifying tags for the ultrasensitive electrochemical detection of procalcitonin. Clin. Biochem. 48(3), 156–161 (2015). https://doi.org/10.1016/j.clinbiochem.2014.09.017
- Z.H. Yang, S. Ren, Y. Zhuo, R. Yuan, Y.Q. Chai, Cu/Mn double-doped CeO2 nanocomposites as signal tags and signal amplifiers for sensitive electrochemical detection of procalcitonin. Anal. Chem. 89(24), 13349–13356 (2017). https://doi.org/10.1021/acs.analchem.7b03502
- C.Y. Chiang, T.T. Huang, C.H. Wang, C.J. Huang, T.H. Tsai et al., Fiber optic nanogold-linked immunosorbent assay for rapid detection of procalcitonin at femtomolar concentration level. Biosens. Bioelectron. 151(1), 111871 (2020). https://doi.org/10.1016/j.bios.2019.111871
- F. Liu, G. Xiang, R. Yuan, X. Chen, F. Luo et al., Procalcitonin sensitive detection based on graphene-gold nanocomposite film sensor platform and single-walled carbon nanohorns/hollow pt chains complex as signal tags. Biosens. Bioelectron. 60(1), 210–217 (2014). https://doi.org/10.1016/j.bios.2014.03.071
- W. Jing, Y. Wang, Y. Yang, Y. Wang, G. Ma et al., Time-resolved digital immunoassay for rapid and sensitive quantitation of procalcitonin with plasmonic imaging. ACS Nano 13(8), 8609–8617 (2019). https://doi.org/10.1021/acsnano.9b02771
- X. Xu, X. Song, R. Nie, Y. Yang, Y. Chen et al., Ultra-sensitive capillary immunosensor combining porous-layer surface modification and biotin-streptavidin nano-complex signal amplification: application for sensing of procalcitonin in serum. Talanta 205(1), 120089 (2019). https://doi.org/10.1016/j.talanta.2019.06.089
- Q. Lin, J. Wu, X. Fang, J. Kong, Washing-free centrifugal microchip fluorescence immunoassay for rapid and point-of-care detection of protein. Anal. Chim. Acta 1118(1), 18–25 (2020). https://doi.org/10.1016/j.aca.2020.04.031
- W.W. Xiong, G.H. Yang, X.C. Wu, J.J. Zhu, Aqueous synthesis of color-tunable CuInS2/ZnS nanocrystals for the detection of human interleukin 6. ACS Appl. Mater. Interfaces 5(16), 8210–8216 (2013). https://doi.org/10.1021/am402328t
- Z. Hao, Y. Pan, W. Shao, Q. Lin, X. Zhao, Graphene-based fully integrated portable nanosensing system for on-line detection of cytokine biomarkers in saliva. Biosens. Bioelectron. 134(1), 16–23 (2019). https://doi.org/10.1016/j.bios.2019.03.053
- U.Y. Lau, S.S. Saxer, J. Lee, E. Bat, H.D. Maynard, Direct write protein patterns for multiplexed cytokine detection from live cells using electron beam lithography. ACS Nano 10(1), 723–729 (2016). https://doi.org/10.1021/acsnano.5b05781
- E. Sánchez-Tirado, C. Salvo, A. González-Cortés, P. Yáñez-Sedeño, F. Langa et al., Electrochemical immunosensor for simultaneous determination of interleukin-1 beta and tumor necrosis factor alpha in serum and saliva using dual screen printed electrodes modified with functionalized double–walled carbon nanotubes. Anal. Chim. Acta 959(1), 66–73 (2017). https://doi.org/10.1016/j.aca.2016.12.034
- B.R. Oh, N.T. Huang, W.Q. Chen, J. Hwan-Seo, P.Y. Chen et al., Integrated nanoplasmonic sensing for cellular functional immunoanalysis using human blood. ACS Nano 8(3), 2667–2676 (2014). https://doi.org/10.1021/nn406370u
- Y. Park, B. Ryu, B.R. Oh, Y. Song, X. Liang et al., Biotunable nanoplasmonic filter on few-layer MoS2 for rapid and highly sensitive cytokine optoelectronic immunosensing. ACS Nano 11(6), 5697–5705 (2017). https://doi.org/10.1021/acsnano.7b01162
- J. Min, M. Nothing, B. Coble, H. Zheng, J. Park et al., Integrated biosensor for rapid and point-of-care sepsis diagnosis. ACS Nano 12(4), 3378–3384 (2018). https://doi.org/10.1021/acsnano.7b08965
- P.Y. Chen, M.T. Chung, W. McHugh, R. Nidetz, Y.W. Li et al., Multiplex serum cytokine immunoassay using nanoplasmonic biosensor microarrays. ACS Nano 9(4), 4173–4181 (2015). https://doi.org/10.1021/acsnano.5b00396
- M.N. Hsu, S.C. Wei, D.T. Phan, Y. Zhang, C.H. Chen, Nano-in-micro smart hydrogel composite for a rapid sensitive immunoassay. Adv. Healthc. Mater. 8(4), e1801277 (2019). https://doi.org/10.1002/adhm.201801277
- H.W. Gu, P.L. Ho, K.W.T. Tsang, L. Wang, B. Xu, Using biofunctional magnetic nanoparticles to capture vancomycin-resistant. J. Am. Chem. Soc. 125, 15702–15703 (2003). https://doi.org/10.1021/ja0359310
- R. Frank, R. Hargreaves, Clinical biomarkers in drug discovery and development. Nat. Rev. Drug Discov. 2(7), 566–580 (2003). https://doi.org/10.1038/nrd1130
- C.F. Oliveira, F.A. Botoni, C.R. Oliveira, C.B. Silva, H.A. Pereira et al., Procalcitonin versus c-reactive protein for guiding antibiotic therapy in sepsis: a randomized trial. Crit. Care Med. 41(10), 2336–2343 (2013). https://doi.org/10.1097/CCM.0b013e31828e969f
- M.K. Masud, J. Na, M. Younus, M.S.A. Hossain, Y. Bando et al., Superparamagnetic nanoarchitectures for disease-specific biomarker detection. Chem. Soc. Rev. 48(24), 5717–5751 (2019). https://doi.org/10.1039/c9cs00174c
- M. Perfezou, A. Turner, A. Merkoci, Cancer detection using nanoparticle-based sensors. Chem. Soc. Rev. 41(7), 2606–2622 (2012). https://doi.org/10.1039/c1cs15134g
- K.K. Jain, Applications of nanobiotechnology in clinical diagnostics. Clin. Chem. 53(11), 2002–2009 (2007). https://doi.org/10.1373/clinchem.2007.090795
- N. Widiarti, J.K. Sae, S. Wahyuni, Synthesis CuO–ZnO nanocomposite and its application as an antibacterial agent. IOP Conf. Ser. Mater. Sci. Eng. 172(1), 012036 (2017). https://doi.org/10.1088/1757-899x/172/1/012036
- P. Schuetz, C. Bretscher, L. Bernasconi, B. Mueller, Overview of procalcitonin assays and procalcitonin-guided protocols for the management of patients with infections and sepsis. Expert Rev. Mol. Diagn. 17(6), 593–601 (2017). https://doi.org/10.1080/14737159.2017.1324299
- C. Wacker, A. Prkno, F.M. Brunkhorst, P. Schlattmann, Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect. Dis. 13(5), 426–435 (2013). https://doi.org/10.1016/s1473-3099(12)70323-7
- M. Stocker, W. van Herk, S. el Helou, S. Dutta, M.S. Fontana et al., Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: a multicentre, randomised controlled trial (neopins). Lancet 390(10097), 871–881 (2017). https://doi.org/10.1016/s0140-6736(17)31444-7
- M. Meisner, Update on procalcitonin measurements. Ann. Lab. Med. 34(4), 263–273 (2014). https://doi.org/10.3343/alm.2014.34.4.263
- E. Mauriz, P. Dey, L.M. Lechuga, Advances in nanoplasmonic biosensors for clinical applications. The Analyst 144(24), 7105–7129 (2019). https://doi.org/10.1039/c9an00701f
- C. Nedeva, J. Menassa, H. Puthalakath, Sepsis: inflammation is a necessary evil. Front. Cell Dev. Biol. 7(1), 108 (2019). https://doi.org/10.3389/fcell.2019.00108
- D. Rittirsch, M.A. Flierl, P.A. Ward, Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 8(10), 776–787 (2008). https://doi.org/10.1038/nri2402
- J.D. Spitzberg, A. Zrehen, X.F. van Kooten, A. Meller, Plasmonic-nanopore biosensors for superior single-molecule detection. Adv. Mater. 31(23), e1900422 (2019). https://doi.org/10.1002/adma.201900422
- Y.C. Yeh, T.H. Huang, S.C. Yang, C.C. Chen, J.Y. Fang, Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances. Front. Chem. 8(1), 286 (2020). https://doi.org/10.3389/fchem.2020.00286
- M.A. Mohamed, Myco-engineered gold nanoparticles from jahnula aquatica coated with ampicillin/amoxicillin and their antibacterial and anticancer activity against cancer cells. Biotechnol. Lett. 42(1), 151–170 (2020). https://doi.org/10.1007/s10529-019-02764-5
- G.B. Hwang, H. Huang, G. Wu, J. Shin, A. Kafizas et al., Photobactericidal activity activated by thiolated gold nanoclusters at low flux levels of white light. Nat. Commun. 11(1), 1207 (2020). https://doi.org/10.1038/s41467-020-15004-6
- D.P. Linklater, V.A. Baulin, X. Le Guevel, J.B. Fleury, E. Hanssen et al., Antibacterial action of nanoparticles by lethal stretching of bacterial cell membranes. Adv. Mater. 32, 2005679 (2020). https://doi.org/10.1002/adma.202005679
- M.A.M. Jahromi, P.S. Zangabad, S.M.M. Basri, K.S. Zangabad, A. Ghamarypour et al., Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliver. Rev. 123(1), 33–64 (2018). https://doi.org/10.1016/j.addr.2017.08.001
- J. Verma, J. Kanoujia, P. Parashar, C.B. Tripathi, S.A. Saraf, Wound healing applications of sericin/chitosan-capped silver nanoparticles incorporated hydrogel. Drug Deliv. Transl. Res. 7(1), 77–88 (2017). https://doi.org/10.1007/s13346-016-0322-y
- L. Rizzello, P.P. Pompa, Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 43(5), 1501–1518 (2014). https://doi.org/10.1039/c3cs60218d
- X. Li, H. Bai, Y. Yang, J. Yoon, S. Wang et al., Supramolecular antibacterial materials for combatting antibiotic resistance. Adv. Mater. 31(5), e1805092 (2019). https://doi.org/10.1002/adma.201805092
- I.R. Scolari, P.L. Paez, M.M. Musri, J.P. Petiti, A. Torres et al., Rifampicin loaded in alginate/chitosan nanoparticles as a promising pulmonary carrier against staphylococcus aureus. Drug Deliv. Transl. Res. 10(5), 1403–1417 (2020). https://doi.org/10.1007/s13346-019-00705-3
- D. Mazzaccaro, R. Ticozzi, S. D’Alessandro, S. Delbue, G. Nano et al., Effect of antibiotic-loaded chitosan nanodroplets on enterococci isolated from chronic ulcers of the lower limbs. Future Microbiol. 15(13), 1227–1236 (2020). https://doi.org/10.2217/fmb-2019-0255
- C. Hu, F. Zhang, L. Long, Q. Kong, R. Luo et al., Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control Release 324(1), 204–217 (2020). https://doi.org/10.1016/j.jconrel.2020.05.010
- L.D. Duceac, G. Calin, L. Eva, C. Marcu, E.R. Bogdan-Goroftei et al., Third-generation cephalosporin-loaded chitosan used to limit microorganisms resistance. Materials 13(21), 4792 (2020). https://doi.org/10.3390/ma13214792
- Z. Li, G. Luo, W.P. Hu, J.L. Hua, S. Geng et al., Mediated drug release from nanovehicles by black phosphorus quantum dots for efficient therapy of chronic obstructive pulmonary disease. Angew. Chem. Int. Ed. 59(1), 20568–20576 (2020). https://doi.org/10.1002/anie.202008379
- E.B. Yahya, F. Jummaat, A.A. Amirul, A.S. Adnan, N.G. Olaiya et al., A review on revolutionary natural biopolymer-based aerogels for antibacterial delivery. Antibiotics 9(10), 648 (2020). https://doi.org/10.3390/antibiotics9100648
- X.X. Wu, Y. Zhang, T. Hu, W.X. Li, Z.L. Li et al., Long-term antibacterial composite via alginate aerogel sustained release of antibiotics and Cu used for bone tissue bacteria infection. Int. J. Biol. Macromol. (2020). https://doi.org/10.1016/j.ijbiomac.2020.11.075
- S. Obuobi, K. Julin, E.G.A. Fredheim, M. Johannessen, N. Skalko-Basnet, Liposomal delivery of antibiotic loaded nucleic acid nanogels with enhanced drug loading and synergistic anti-inflammatory activity against S. aureus intracellular infections. J. Control. Release 324(1), 620–632 (2020). https://doi.org/10.1016/j.jconrel.2020.06.002
- E. Teirlinck, A. Barras, J. Liu, J.C. Fraire, T. Lajunen et al., Exploring light-sensitive nanocarriers for simultaneous triggered antibiotic release and disruption of biofilms upon generation of laser-induced vapor nanobubbles. Pharmaceutics 11(5), 201 (2019). https://doi.org/10.3390/pharmaceutics11050201
- K.A. Vandera, P. Picconi, M. Valero, G. Gonzalez-Gaitano, A. Woods et al., Antibiotic-in-cyclodextrin-in-liposomes: formulation development and interactions with model bacterial membranes. Mol. Pharmaceut. 17(7), 2354–2369 (2020). https://doi.org/10.1021/acs.molpharmaceut.0c00096
- X. Pang, Q. Xiao, Y. Cheng, E. Ren, L. Lian et al., Bacteria-responsive nanoliposomes as smart sonotheranostics for multidrug resistant bacterial infections. ACS Nano 13(2), 2427–2438 (2019). https://doi.org/10.1021/acsnano.8b09336
- Y. Wu, Z. Song, H. Wang, H. Han, Endogenous stimulus-powered antibiotic release from nanoreactors for a combination therapy of bacterial infections. Nat. Commun. 10(1), 4464 (2019). https://doi.org/10.1038/s41467-019-12233-2
- G. Yang, S. Chen, J. Zhang, Bioinspired and biomimetic nanotherapies for the treatment of infectious diseases. Front. Pharmacol. 10(1), 751 (2019). https://doi.org/10.3389/fphar.2019.00751
- W. Gao, L. Zhang, Coating nanoparticles with cell membranes for targeted drug delivery. J. Drug Target. 23(7–8), 619–626 (2015). https://doi.org/10.3109/1061186X.2015.1052074
- W. Huang, Q. Zhang, W. Li, M. Yuan, J. Zhou et al., Development of novel nanoantibiotics using an outer membrane vesicle-based drug efflux mechanism. J. Control. Release 317(1), 1–22 (2020). https://doi.org/10.1016/j.jconrel.2019.11.017
- F. Gao, L. Xu, B. Yang, F. Fan, L. Yang, Kill the real with the fake: Eliminate intracellular staphylococcus aureus using nanoparticle coated with its extracellular vesicle membrane as active-targeting drug carrier. ACS Infect. Dis. 5(2), 218–227 (2019). https://doi.org/10.1021/acsinfecdis.8b00212
- R. Pascale, M. Giannella, M. Bartoletti, P. Viale, F. Pea, Use of meropenem in treating carbapenem-resistant enterobacteriaceae infections. Expert Rev. Anti-Infe. Therapy 17(10), 819–827 (2019). https://doi.org/10.1080/14787210.2019.1673731
- F.M. Brunkhorst, M. Oppert, G. Marx, F. Bloos, K. Ludewig et al., Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA 307(22), 2390–2399 (2012). https://doi.org/10.1001/jama.2012.5833
- J.A. Roberts, M.H. Abdul-Aziz, J.S. Davis, J.M. Dulhunty, M.O. Cotta et al., Continuous versus intermittent b-lactam infusion in severe sepsis: a meta-analysis of individual patient data from randomized trials. Am. J. Resp. Crit. Care 194(6), 681–691 (2016). https://doi.org/10.1164/rccm.201601-0024OC
- P.N.A. Harris, P.A. Tambyah, D.C. Lye, Y. Mo, T.H. Lee et al., N. the Australasian Society for Infectious Disease Clinical Research. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with e coli or klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. Jama 320(10), 984–994 (2018). https://doi.org/10.1001/jama.2018.12163
- A. Abdelkader, M.A. El-Mokhtar, O. Abdelkader, M.A. Hamad, M. Elsabahy et al., Ultrahigh antibacterial efficacy of meropenem-loaded chitosan nanoparticles in a septic animal model. Carbohyd. Polym. 174(1), 1041–1050 (2017). https://doi.org/10.1016/j.carbpol.2017.07.030
- J. Ndayishimiye, A. Popat, M. Blaskovich, J.R. Falconer, Formulation technologies and advances for oral delivery of novel nitroimidazoles and antimicrobial peptides. J. Control. Release (2020). https://doi.org/10.1016/j.jconrel.2020.05.002
- A.C. Saude, A.S. Ombredane, O.N. Silva, J.A. Barbosa, S.E. Moreno et al., Clavanin bacterial sepsis control using a novel methacrylate nanocarrier. Int. J. Nanomed. 9(1), 5055–5069 (2014). https://doi.org/10.2147/IJN.S66300
- C. Falciani, F. Zevolini, J. Brunetti, G. Riolo, R. Gracia et al., Antimicrobial peptide-loaded nanoparticles as inhalation therapy for pseudomonas aeruginosa infections. Int. J. Nanomed. 15(1), 1117–1128 (2020). https://doi.org/10.2147/IJN.S218966
- N. Mookherjee, M.A. Anderson, H.P. Haagsman, D.J. Davidson, Antimicrobial host defence peptides: functions and clinical potential. Nat. Rev. Drug Discov. 19(5), 311–332 (2020). https://doi.org/10.1038/s41573-019-0058-8
- A.J. Beevers, A.M. Dixon, Helical membrane peptides to modulate cell function. Chem. Soc. Rev. 39(6), 2146–2157 (2010). https://doi.org/10.1039/b912944h
- T. Ganz, Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3(9), 710–720 (2003). https://doi.org/10.1038/nri1180
- B.P. Lazzaro, M. Zasloff, J. Rolff, Antimicrobial peptides: application informed by evolution. Science 368(6490), 5480 (2020). https://doi.org/10.1126/science.aau5480
- Y. Scindia, E. Wlazlo, J. Leeds, V. Loi, J. Ledesma et al., Protective role of hepcidin in polymicrobial sepsis and acute kidney injury. Front. Pharm. 10(1), 615 (2019). https://doi.org/10.3389/fphar.2019.00615
- Y.H. Huang, Y.L. Yang, M.M. Tiao, H.C. Kuo, L.T. Huang et al., Hepcidin protects against lipopolysaccharide-induced liver injury in a mouse model of obstructive jaundice. Peptides 35(2), 212–217 (2012). https://doi.org/10.1016/j.peptides.2012.03.032
- N.H. Salzman, D. Ghosh, K.M. Huttner, Y. Patersonk, C.L. Bevins, Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422(6931), 522–526 (2003). https://doi.org/10.1038/nature01520
- E. de Leeuw, S.R. Burks, X. Li, J.P. Kao, W. Lu, Structure-dependent functional properties of human defensin 5. FEBS Lett. 581(3), 515–520 (2007). https://doi.org/10.1016/j.febslet.2006.12.036
- C. Wang, M. Shen, N. Gohain, W.D. Tolbert, F. Chen et al., Design of a potent antibiotic peptide based on the active region of human defensin 5. J. Med. Chem. 58(7), 3083–3093 (2015). https://doi.org/10.1021/jm501824a
- A. de Breij, M. Riool, R.A. Cordfunke, N. Malanovic, L. de Boer et al., The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 10, 4044 (2018). https://doi.org/10.1126/scitranslmed.aan4044
- L. Liu, K. Xu, H. Wang, P.K. Tan, W. Fan et al., Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat. Nanotechnol. 4(7), 457–463 (2009). https://doi.org/10.1038/nnano.2009.153
- B. Mathew, R. Nagaraj, Antimicrobial activity of human alpha-defensin 5 and its linear analogs: N-terminal fatty acylation results in enhanced antimicrobial activity of the linear analogs. Peptides 71, 128–140 (2015). https://doi.org/10.1016/j.peptides.2015.07.009
- J.J. Lee, K.J. Jeong, M. Hashimoto, A.H. Kwon, A. Rwei et al., Synthetic ligand-coated magnetic nanoparticles for microfluidic bacterial separation from blood. Nano Lett. 14(1), 1–5 (2014). https://doi.org/10.1021/nl3047305
- X. Hou, X. Zhang, W. Zhao, C. Zeng, B. Deng et al., Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol. 15(1), 41–46 (2020). https://doi.org/10.1038/s41565-019-0600-1
- J. Zhou, G. Yu, F. Huang, Supramolecular chemotherapy based on host-guest molecular recognition: a novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 46(22), 7021–7053 (2017). https://doi.org/10.1039/c6cs00898d
- I.K. Herrmann, M. Urner, S. Graf, C.M. Schumacher, B. Roth-Z’graggen et al., Endotoxin removal by magnetic separation-based blood purification. Adv. Healthc. Mater. 2(6), 829–835 (2013). https://doi.org/10.1002/adhm.201200358
- T. Liu, B. Xiao, F. Xiang, J. Tan, Z. Chen et al., Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 11(1), 2788 (2020). https://doi.org/10.1038/s41467-020-16544-7
- M. Soh, D.W. Kang, H.G. Jeong, D. Kim, D.Y. Kim et al., Ceria-zirconia nanoparticles as an enhanced multi-antioxidant for sepsis treatment. Angew. Chem. Int. Ed. 56(38), 11399–11403 (2017). https://doi.org/10.1002/anie.201704904
- V. Selvaraj, N.D. Manne, R. Arvapalli, K.M. Rice, G. Nandyala et al., Effect of cerium oxide nanoparticles on sepsis induced mortality and NF-kappaB signaling in cultured macrophages. Nanomedicine 10(8), 1275–1288 (2015). https://doi.org/10.2217/nnm.14.205
- S. Asano, R. Arvapalli, N.D. Manne, M. Maheshwari, B. Ma et al., Cerium oxide nanoparticle treatment ameliorates peritonitis-induced diaphragm dysfunction. Int. J. Nanomed. 10(1), 6215–6225 (2015). https://doi.org/10.2147/IJN.S89783
- S.K. Rajendrakumar, V. Revuri, M. Samidurai, A. Mohapatra, J.H. Lee et al., Peroxidase-mimicking nanoassembly mitigates lipopolysaccharide-induced endotoxemia and cognitive damage in the brain by impeding inflammatory signaling in macrophages. Nano Lett. 18(10), 6417–6426 (2018). https://doi.org/10.1021/acs.nanolett.8b02785
- S. Thamphiwatana, P. Angsantikul, T. Escajadillo, Q. Zhang, J. Olson et al., Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc. Natl. Acad. Sci. USA 114(43), 11488–11493 (2017). https://doi.org/10.1073/pnas.1714267114
- R. Molinaro, A. Pasto, C. Corbo, F. Taraballi, F. Giordano et al., Macrophage-derived nanovesicles exert intrinsic anti-inflammatory properties and prolong survival in sepsis through a direct interaction with macrophages. Nanoscale 11(28), 13576–13586 (2019). https://doi.org/10.1039/c9nr04253a
- S. Shen, F. Han, A. Yuan, L. Wu, J. Cao et al., Engineered nanoparticles disguised as macrophages for trapping lipopolysaccharide and preventing endotoxemia. Biomaterials 189(1), 60–68 (2019). https://doi.org/10.1016/j.biomaterials.2018.10.029
- V. Chhabria, S. Beeton, Development of nanosponges from erythrocyte ghosts for removal of streptolysin-o from mammalian blood. Nanomedicine 11(21), 2797–2807 (2016). https://doi.org/10.2217/nnm-2016-0180
- Y. Chen, Y. Zhang, M. Chen, J. Zhuang, R.H. Fang et al., Biomimetic nanosponges suppress in vivo lethality induced by the whole secreted proteins of pathogenic bacteria. Small 15(6), e1804994 (2019). https://doi.org/10.1002/smll.201804994
- G. Kibria, E.K. Ramos, Y. Wan, D.R. Gius, H. Liu, Exosomes as a drug delivery system in cancer therapy: potential and challenges. Mol. Pharmaceut. 15(9), 3625–3633 (2018). https://doi.org/10.1021/acs.molpharmaceut.8b00277
- N. Terrasini, V. Lionetti, Exosomes in critical illness. Crit. Care Med. 45(6), 1054–1060 (2017). https://doi.org/10.1097/CCM.0000000000002328
- J. Gao, S. Wang, Z. Wang, High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVS) for anti-inflammation therapy. Biomaterials 135, 62–73 (2017). https://doi.org/10.1016/j.biomaterials.2017.05.003
- K.S. Park, K. Svennerholm, G.V. Shelke, E. Bandeira, C. Lasser et al., Mesenchymal stromal cell-derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via il-10. Stem Cell Res. Ther. 10(1), 231 (2019). https://doi.org/10.1186/s13287-019-1352-4
- W. Lee, J. Seo, S. Kwak, E.J. Park, D.H. Na et al., A double-chambered protein nanocage loaded with thrombin receptor agonist peptide (TRAP) and gamma-carboxyglutamic acid of protein c (PC-Gla) for sepsis treatment. Adv. Mater. 27(42), 6637–6643 (2015). https://doi.org/10.1002/adma.201503093
- C.Y. Zhang, X. Dong, J. Gao, W. Lin, Z. Liu et al., Nanoparticle-induced neutrophil apoptosis increasessurvival in sepsis and alleviates neurologicaldamage in stroke. Sci. Adv. 5, 7964 (2019). https://doi.org/10.1126/sciadv.aax7964
- Y. Xin, M. Huang, W.W. Guo, Q. Huang, L.Z. Zhang et al., Nano-based delivery of RNAI in cancer therapy. Mol. Cancer 16(1), 134 (2017). https://doi.org/10.1186/s12943-017-0683-y
- H. He, N. Zheng, Z. Song, K.H. Kim, C. Yao et al., Suppression of hepatic inflammation via systemic sirna delivery by membrane-disruptive and endosomolytic helical polypeptide hybrid nanoparticles. ACS Nano 10(2), 1859–1870 (2016). https://doi.org/10.1021/acsnano.5b05470
- R. Penalva, C.J. Gonzalez-Navarro, C. Gamazo, I. Esparza, J.M. Irache, Zein nanoparticles for oral delivery of quercetin: pharmacokinetic studies and preventive anti-inflammatory effects in a mouse model of endotoxemia. Nanomed. Nanotechnol. 13(1), 103–110 (2017). https://doi.org/10.1016/j.nano.2016.08.033
- S. Mura, J. Nicolas, P. Couvreur, Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12(11), 991–1003 (2013). https://doi.org/10.1038/nmat3776
- L.M. Casey, S. Kakade, J.T. Decker, J.A. Rose, K. Deans et al., Cargo-less nanoparticles program innate immune cell responses to toll-like receptor activation. Biomaterials 218, 119333 (2019). https://doi.org/10.1016/j.biomaterials.2019.119333
- S. Spence, M.K. Greene, F. Fay, E. Hams, S.P. Saunders et al., Targeting siglecs with a sialic acid–decoratednanoparticle abrogates inflammation. Sci. Transl. Med. 7(303), 303ra140 (2015). https://doi.org/10.1126/scitranslmed.aab3459
- G. Li-Volti, T. Musumeci, R. Pignatello, P. Murabito, I. Barbagallo et al., Antioxidant potential of different melatonin-loaded nanomedicines in an experimental model of sepsis. Exp. Biol. Med. 237(6), 670–677 (2012). https://doi.org/10.1258/ebm.2012.011425
- S. Zhang, J. Ermann, M.D. Succi, A. Zhou, M.J. Hamilton et al., An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci. Transl. Med. 7(300), 300128 (2015). https://doi.org/10.1126/scitranslmed.aaa5657
- N. Joshi, J. Yan, S. Levy, S. Bhagchandani, K.V. Slaughter et al., Towards an arthritis flare-responsive drug delivery system. Nat. Commun. 9(1), 1275 (2018). https://doi.org/10.1038/s41467-018-03691-1
- S.B. Lim, I. Rubinstein, R.T. Sadikot, J.E. Artwohl, H. Onyuksel, A novel peptide nanomedicine against acute lung injury: Glp-1 in phospholipid micelles. Pharm. Res. 28(3), 662–672 (2011). https://doi.org/10.1007/s11095-010-0322-4
- M.C. Ferrer, V.V. Shuvaev, B.J. Zern, R.J. Composto, V.R. Muzykantov et al., ICAM-1 targeted nanogels loaded with dexamethasone alleviate pulmonary inflammation. PLoS ONE 9(7), e102329 (2014). https://doi.org/10.1371/journal.pone.0102329
- E.B. Okeke, C. Louttit, C. Fry, A.H. Najafabadi, K. Han et al., Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials 238(1), 119836 (2020). https://doi.org/10.1016/j.biomaterials.2020.119836
- C.Y. Zhang, J. Gao, Z. Wang, Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv. Mater. 30(43), e1803618 (2018). https://doi.org/10.1002/adma.201803618
- Y. Yang, Y. Ding, B. Fan, Y. Wang, Z. Mao et al., Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J. Control. Release 321, 463–474 (2020). https://doi.org/10.1016/j.jconrel.2020.02.030
- W. Lee, E.J. Park, G. Min, J. Choi, D.H. Na et al., Dual functioned pegylated phospholipid micelles containing cationic antimicrobial decapeptide for treating sepsis. Theranostics 7(15), 3759–3767 (2017). https://doi.org/10.7150/thno.20734
- L. Tang, Z. Wang, Q. Mu, Z. Yu, O. Jacobson et al., Targeting neutrophils for enhanced cancer theranostics. Adv. Mater. 32(33), e2002739 (2020). https://doi.org/10.1002/adma.202002739
- S. Wang, J. Lv, S. Meng, J. Tang, L. Nie, Recent advances in nanotheranostics for treat-to-target of rheumatoid arthritis. Adv. Healthc. Mater. 9(6), e1901541 (2020). https://doi.org/10.1002/adhm.201901541
- B. Ma, H. Xu, W. Zhuang, Y. Wang, G. Li et al., Reactive oxygen species responsive theranostic nanoplatform for two-photon aggregation-induced emission imaging and therapy of acute and chronic inflammation. ACS Nano 14(5), 5862–5873 (2020). https://doi.org/10.1021/acsnano.0c01012
- E.V. Fuior, C.A. Mocanu, M. Deleanu, G. Voicu, M. Anghelache et al., Evaluation of VCAM-1 targeted naringenin/indocyanine green-loaded lipid nanoemulsions as theranostic nanoplatforms in inflammation. Pharmaceutics 12(11), 1066 (2020). https://doi.org/10.3390/pharmaceutics12111066
- D. Mao, F. Hu, S. Ji, W. Wu, D. Ding et al., Metal-organic-framework-assisted in vivo bacterial metabolic labeling and precise antibacterial therapy. Adv. Mater. 30(18), e1706831 (2018). https://doi.org/10.1002/adma.201706831
- C. Shi, X. Wang, L. Wang, Q. Meng, D. Guo et al., A nanotrap improves survival in severe sepsis by attenuating hyperinflammation. Nat. Commun. 11(1), 3384 (2020). https://doi.org/10.1038/s41467-020-17153-0
- M.I. Setyawati, C.Y. Tay, D. Docter, R.H. Stauber, D.T. Leong, Understanding and exploiting nanoparticles’ intimacy with the blood vessel and blood. Chem. Soc. Rev. 44(22), 8174–8199 (2015). https://doi.org/10.1039/c5cs00499c
- M. Wang, J. Li, S. Dong, X. Cai, A. Simaiti et al., Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction. Part. Fibre Toxicol. 17(1), 23 (2020). https://doi.org/10.1186/s12989-020-00353-3
References
M. Singer, C.S. Deutschman, C.W. Seymour, M. Shankar-Hari, D. Annane et al., The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315(8), 801–810 (2016). https://doi.org/10.1001/jama.2016.0287
C. Fleischmann, A. Scherag, N.K. Adhikari, C.S. Hartog, T. Tsaganos et al., International forum of acute care. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Resp. Care 193(3), 259–272 (2016). https://doi.org/10.1164/rccm.201504-0781OC
D.C. Angus, W.T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo et al., Epidemiology of severe sepsis in the united states: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29(7), 1303–1310 (2001). https://doi.org/10.1097/00003246-00107000-00002
F.B. Mayr, S. Yende, D.C. Angus, Epidemiology of severe sepsis. Virulence 5(1), 4–11 (2014). https://doi.org/10.4161/viru.27372
D.J. Funk, J.E. Parrillo, A. Kumar, Sepsis and septic shock: a history. Crit. Care Clin. 25(1), 83–101 (2009). https://doi.org/10.1016/j.ccc.2008.12.003
SCCM, American college of chest physicians/society of critical care medicine consensus conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 20(6), 864–874 (1992). https://doi.org/10.1097/00003246-199206000-00025
M.M. Levy, M.P. Fink, J.C. Marshall, E. Abraham, D. Angus et al., 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit. Care Med. 31(4), 1250–1256 (2003). https://doi.org/10.1097/01.CCM.0000050454.01978.3B
V. Liu, G.J. Escobar, J.D. Greene, J. Soule, A. Whippy et al., Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 312(1), 90–92 (2014). https://doi.org/10.1001/jama.2014.5804
W.T. Linde-Zwirble, D.C. Angus, Severe sepsis epidemiology: Sampling, selection, and society. Crit. Care 8(4), 222–226 (2004). https://doi.org/10.1186/cc2917
M. Cecconi, L. Evans, M. Levy, A. Rhodes, Sepsis and septic shock. Lancet 392(10141), 75–87 (2018). https://doi.org/10.1016/s0140-6736(18)30696-2
B. Cheng, G. Xie, S. Yao, X. Wu, Q. Guo et al., Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in china. Crit. Care Med. 35(11), 2538–2546 (2007). https://doi.org/10.1097/01.CCM.0000284492.30800.00
K.E. Rudd, S.C. Johnson, K.M. Agesa, K.A. Shackelford, D. Tsoi et al., Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the global burden of disease study. Lancet 395(10219), 200–211 (2020). https://doi.org/10.1016/s0140-6736(19)32989-7
T. van der Poll, F.L. van de Veerdonk, B.P. Scicluna, M.G. Netea, The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 17(7), 407–420 (2017). https://doi.org/10.1038/nri.2017.36
M.P. Fink, H.S. Warren, Strategies to improve drug development for sepsis. Nat. Rev. Drug Discov. 13(10), 741–758 (2014). https://doi.org/10.1038/nrd4368
K.N. Iskander, M.F. Osuchowski, D.J. Stearns-Kurosawa, S. Kurosawa, D. Stepien et al., Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Phys. Rev. 93(3), 1247–1288 (2013). https://doi.org/10.1152/physrev.00037.2012
R.S. Hotchkiss, L.L. Moldawer, S.M. Opal, K. Reinhart, I.R. Turnbull et al., Sepsis and septic shock. Nat. Rev. Dis. Primers 2(1), 16045 (2016). https://doi.org/10.1038/nrdp.2016.45
F. Venet, G. Monneret, Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 14(2), 121–137 (2018). https://doi.org/10.1038/nrneph.2017.165
J.C. Marshall, Why have clinical trials in sepsis failed? Trends Mol. Med. 20(4), 195–203 (2014). https://doi.org/10.1016/j.molmed.2014.01.007
J.L. Halbach, A.W. Wang, D. Hawisher, D.M. Cauvi, R.E. Lizardo et al., Why antibiotic treatment is not enough for sepsis resolution: an evaluation in an experimental animal model. Infect. Immun. 85(12), e0066400617 (2017). https://doi.org/10.1128/IAI.00664-17
J.C. Hou, Q. Chen, K. Zhang, B.L. Cheng, G.H. Xie et al., Sphingosine 1-phosphate receptor 2 signaling suppresses macrophage phagocytosis and impairs host defense against sepsis. Anesthesiology 123(2), 409–422 (2015). https://doi.org/10.1097/ALN.0000000000000725
F. Song, J. Hou, Z. Chen, B. Cheng, R. Lei et al., Sphingosine-1-phosphate receptor 2 signaling promotes caspase-11-dependent macrophage pyroptosis and worsens escherichia coli sepsis outcome. Anesthesiology 129(2), 311–320 (2018). https://doi.org/10.1097/ALN.0000000000002196
F. Niessen, F. Schaffner, C. Furlan-Freguia, R. Pawlinski, G. Bhattacharjee et al., Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature 452(7187), 654–658 (2008). https://doi.org/10.1038/nature06663
J. Hou, Q. Chen, X. Wu, D. Zhao, H. Reuveni et al., S1PR3 signaling drives bacterial killing and is required for survival in bacterial sepsis. Am. J. Resp. Crit Care 196(12), 1559–1570 (2017). https://doi.org/10.1164/rccm.201701-0241OC
A. Bouchon, F. Facchetti, M.A. Weigand, M. Colonna, Trem-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410(6832), 1103–1107 (2001). https://doi.org/10.1038/35074114
Q. Chen, K. Zhang, Y. Jin, T. Zhu, B. Cheng et al., Triggering receptor expressed on myeloid cells-2 protects against polymicrobial sepsis by enhancing bacterial clearance. Am. J. Resp. Crit. Care 188(2), 201–212 (2013). https://doi.org/10.1164/rccm.201211-1967OC
Q. Chen, Y. Jin, K. Zhang, H. Li, W. Chen et al., Alarmin HNP-1 promotes pyroptosis and IL-1beta release through different roles of NLRP3 inflammasome via P2X7 in LPS-primed macrophages. Innate Immun. 20(3), 290–300 (2014). https://doi.org/10.1177/1753425913490575
Q. Chen, Y. Yang, J. Hou, Q. Shu, Y. Yin et al., Increased gene copy number of defa1/defa3 worsens sepsis by inducing endothelial pyroptosis. Proc. Natl. Acad. Sci. USA 116(8), 3161–3170 (2019). https://doi.org/10.1073/pnas.1812947116
N. Arulkumaran, M.L. Sixma, S. Pollen, E. Ceravola, E. Jentho et al., P2X7 receptor antagonism ameliorates renal dysfunction in a rat model of sepsis. Phys. Rep. 6(5), e13622 (2018). https://doi.org/10.14814/phy2.13622
X.W. Qian, T. Numata, K. Zhang, C.X. Li, J.C. Hou et al., Transient receptor potential melastatin 2 protects mice against polymicrobial sepsis by enhancing bacterial clearance. Anesthesiology 121(2), 336–351 (2014). https://doi.org/10.1097/ALN.0000000000000275
Z. Zhang, P. Cui, K. Zhang, Q. Chen, X. Fang, Transient receptor potential melastatin 2 regulates phagosome maturation and is required for bacterial clearance in escherichia coli sepsis. Anesthesiology 126(1), 128–139 (2017). https://doi.org/10.1097/ALN.0000000000001430
L. Wang, T.-M. Fu, Y. Zhou, S. Xia, A. Greka et al., Structures and gating mechanism of human TRPM2. Science 362(eaav6421), 4809 (2018). https://doi.org/10.1126/science.aav4809
H.R. Schmidt, S. Zheng, E. Gurpinar, A. Koehl, A. Manglik et al., Crystal structure of the human sigma1 receptor. Nature 532(7600), 527–530 (2016). https://doi.org/10.1038/nature17391
D.A. Rosen, S.M. Seki, A. Fernández-Castañeda, R.M. Beiter, J.D. Eccles et al., Modulation of the sigma-1 receptor–IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci. Transl. Med. 11, eaau5266 (2019). https://doi.org/10.1126/scitranslmed.aau5266
A. Rhodes, L.E. Evans, W. Alhazzani, M.M. Levy, M. Antonelli et al., Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intens. Care Med. 43(3), 304–377 (2017). https://doi.org/10.1007/s00134-017-4683-6
C.W. Seymour, F. Gesten, H.C. Prescott, M.E. Friedrich, T.J. Iwashyna et al., Time to treatment and mortality during mandated emergency care for sepsis. N. Engl. J. Med. 376(23), 2235–2244 (2017). https://doi.org/10.1056/NEJMoa1703058
B.-T. Huynh, M. Padget, B. Garin, E. Delarocque-Astagneau, D. Guillemot, Bacterial neonatal sepsis and antibiotic resistance in low-income countries. Lancet 387(10018), 533–534 (2016). https://doi.org/10.1016/s0140-6736(16)00220-8
K.E. Drexler, Molecular engineering: an approach to the development of general capabilities for molecular manipulation. Proc. Natl. Acad. Sci. USA 78(9), 5275–5278 (1981). https://doi.org/10.1073/pnas.78.9.5275
R.T. Sadikot, The potential role of nano- and micro-technology in the management of critical illnesses. Adv. Drug Deliver. Rev. 77(1), 27–31 (2014). https://doi.org/10.1016/j.addr.2014.07.004
J. Shi, P.W. Kantoff, R. Wooster, O.C. Farokhzad, Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 17(1), 20–37 (2017). https://doi.org/10.1038/nrc.2016.108
W. Jiang, H. Yuan, C.K. Chan, C.A. von Roemeling, Z. Yan et al., Lessons from immuno-oncology: a new era for cancer nanomedicine? Nat. Rev. Drug Discov. 16(6), 369–370 (2017). https://doi.org/10.1038/nrd.2017.34
M.E. Lobatto, V. Fuster, Z.A. Fayad, W.J. Mulder, Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 10(11), 835–852 (2011). https://doi.org/10.1038/nrd3578
O. Veiseh, B.C. Tang, K.A. Whitehead, D.G. Anderson, R. Langer, Managing diabetes with nanomedicine: challenges and opportunities. Nat. Rev. Drug Discov. 14(1), 45–57 (2015). https://doi.org/10.1038/nrd4477
A.D. Smith, Big moment for nanotech: oncology therapeutics poised for a leap (2013)
G. Luo, Q. Yang, B. Yao, Y. Tian, R. Hou et al., SLP-coated liposomes for drug delivery and biomedical applications: potential and challenges. Int. J. Nanomed. 14(1), 1359–1383 (2019). https://doi.org/10.2147/IJN.S189935
W. Wang, A. Shao, S. Feng, M. Ding, G. Luo, Physicochemical characterization and gastrointestinal adhesion of s-layer proteins-coating liposomes. Int. J. Pharmaceut. 529(1–2), 227–237 (2017). https://doi.org/10.1016/j.ijpharm.2017.07.006
A. Bernkop-Schnurch, A. Jalil, Do drug release studies from sedds make any sense? J. Control. Release 271(1), 55–59 (2018). https://doi.org/10.1016/j.jconrel.2017.12.027
C. Kinnear, T.L. Moore, L. Rodriguez-Lorenzo, B. Rothen-Rutishauser, A. Petri-Fink, Form follows function: nanoparticle shape and its implications for nanomedicine. Chem. Rev. 117(17), 11476–11521 (2017). https://doi.org/10.1021/acs.chemrev.7b00194
H. Wang, H. Xie, J. Wang, J. Wu, X. Ma et al., Self-assembling prodrugs by precise programming of molecular structures that contribute distinct stability, pharmacokinetics, and antitumor efficacy. Adv. Funct. Mater. 25(31), 4956–4965 (2015). https://doi.org/10.1002/adfm.201501953
J. Wan, Y. Qiao, X. Chen, J. Wu, L. Zhou et al., Structure-guided engineering of cytotoxic cabazitaxel for an adaptive nanoparticle formulation: Enhancing the drug safety and therapeutic efficacy. Adv. Funct. Mater. 28(52), 1804229 (2018). https://doi.org/10.1002/adfm.201804229
S.A. Anuj, H.P. Gajera, D.G. Hirpara, B.A. Golakiya, Bactericidal assessment of nano-silver on emerging and re-emerging human pathogens. J. Trace Elem. Med. Biol. 51, 219–225 (2019). https://doi.org/10.1016/j.jtemb.2018.04.028
Y. Li, Y. Chang, X. Lian, L. Zhou, Z. Yu et al., Silver nanoparticles for enhanced cancer theranostics: in vitro and in vivo perspectives. J. Biomed. Nanotechnol. 14(9), 1515–1542 (2018). https://doi.org/10.1166/jbn.2018.2614
J.R. Melamed, R.S. Riley, D.M. Valcourt, E.S. Day, Using gold nanoparticles to disrupt the tumor microenvironment: an emerging therapeutic strategy. ACS Nano 10(12), 10631–10635 (2016). https://doi.org/10.1021/acsnano.6b07673
W. Gao, Y. Xiong, Q. Li, H. Yang, Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front. Physiol. 8(1), 508 (2017). https://doi.org/10.3389/fphys.2017.00508
P. Cui, X. Fang, Pathogenesis of infection in surgical patients. Curr. Opin. Crit. Care 21(4), 343–350 (2015). https://doi.org/10.1097/MCC.0000000000000227
R. Lei, J. Hou, Q. Chen, W. Yuan, B. Cheng et al., Self-assembling myristoylated human alpha-defensin 5 as a next-generation nanobiotics potentiates therapeutic efficacy in bacterial infection. ACS Nano 12, 5284–5296 (2018). https://doi.org/10.1021/acsnano.7b09109
A. Balakrishnan, P. DasSarma, O. Bhattacharjee, J.M. Kim, S. DasSarma et al., Halobacterial nano vesicles displaying murine bactericidal permeability-increasing protein rescue mice from lethal endotoxic shock. Sci. Rep. 6, 33679 (2016). https://doi.org/10.1038/srep33679
F.H. Liao, T.H. Wu, Y.T. Huang, W.J. Lin, C.J. Su et al., Subnanometer gold clusters adhere to lipid a for protection against endotoxin-induced sepsis. Nano Lett. 18(5), 2864–2869 (2018). https://doi.org/10.1021/acs.nanolett.7b05464
J.L. Perry, K.P. Herlihy, M. Napier, J.M. Desimon, Print: a novel platform toward shape and size specific nanoparticle theranostics. Acc. Chem. Res. 44(10), 990–998 (2011). https://doi.org/10.1021/ar2000315
S. Mura, P. Couvreur, Nanotheranostics for personalized medicine. Adv. Drug Deliver. Rev. 64(13), 1394–1416 (2012). https://doi.org/10.1016/j.addr.2012.06.006
D. Peer, J.M. Karp, S. Hong, O.C. Farokhzad, R. Margalit et al., Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760 (2007). https://doi.org/10.1038/nnano.2007.387
B.R. Smith, S.S. Gambhir, Nanomaterials for in vivo imaging. Chem. Rev. 117(3), 901–986 (2017). https://doi.org/10.1021/acs.chemrev.6b00073
F.M. Kievit, M. Zhang, Cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers. Adv. Mater. 23(36), H217-247 (2011). https://doi.org/10.1002/adma.201102313
W.L. Tang, W.H. Tang, S.D. Li, Cancer theranostic applications of lipid-based nanoparticles. Drug Discov. Today 23(5), 1159–1166 (2018). https://doi.org/10.1016/j.drudis.2018.04.007
Y. Liu, P. Bhattarai, Z. Dai, X. Chen, Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 48(7), 2053–2108 (2019). https://doi.org/10.1039/c8cs00618k
S. Mohamed, S. Veeranarayanan, T. Maekawa, S. Kumar, External stimulus responsive inorganic nanomaterials for cancer theranostics. Adv. Drug Deliver. Rev. 138(1), 18–40 (2019). https://doi.org/10.1016/j.addr.2018.10.007
P. Jagtap, V. Sritharan, S. Gupta, Nanotheranostic approaches for management of bloodstream bacterial infections. Nanomed-Nanotechnol. 13(1), 329–341 (2017). https://doi.org/10.1016/j.nano.2016.09.005
M.D. Howard, E.D. Hood, B. Zern, V.V. Shuvaev, T. Grosser et al., Nanocarriers for vascular delivery of anti-inflammatory agents. Annu. Rev. Pharmacol. 54(1), 205–226 (2014). https://doi.org/10.1146/annurev-pharmtox-011613-140002
A. Kumar, R.K. Chaudhary, R. Singh, S.P. Singh, S.Y. Wang et al., Nanotheranostic applications for detection and targeting neurodegenerative diseases. Front. Neurosci. 14(1), 305 (2020). https://doi.org/10.3389/fnins.2020.00305
F. Liu, L. Lin, Y. Zhang, Y. Wang, S. Sheng et al., A tumor-microenvironment-activated nanozyme-mediated theranostic nanoreactor for imaging-guided combined tumor therapy. Adv. Mater. 31(40), e1902885 (2019). https://doi.org/10.1002/adma.201902885
C.F. Markwalter, A.G. Kantor, C.P. Moore, K.A. Richardson, D.W. Wright, Inorganic complexes and metal-based nanomaterials for infectious disease diagnostics. Chem. Rev. 119(2), 1456–1518 (2019). https://doi.org/10.1021/acs.chemrev.8b00136
B. Yang, Y. Chen, J. Shi, Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 119(8), 4881–4985 (2019). https://doi.org/10.1021/acs.chemrev.8b00626
R. Eivazzadeh-Keihan, E. Bahojb-Noruzi, K. Khanmohammadi-Chenab, A. Jafari, F. Radinekiyan et al., Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 14, 1687–1714 (2020). https://doi.org/10.1002/term.3131
G. Yang, S.Z.F. Phua, A.K. Bindra, Y. Zhao, Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv. Mater. 31(10), e1805730 (2019). https://doi.org/10.1002/adma.201805730
H.P. Lee, A.K. Gaharwar, Light-responsive inorganic biomaterials for biomedical applications. Adv. Sci. 7(17), 2000863 (2020). https://doi.org/10.1002/advs.202000863
J.H. Lee, H.Y. Cho, H.K. Choi, J.Y. Lee, J.W. Choi, Application of gold nanoparticle to plasmonic biosensors. Int. J. Mol. Sci. 19(7), 2021 (2018). https://doi.org/10.3390/ijms19072021
N. Wang, H. Dai, L. Sai, H. Ma, M. Lin, Copper ion-assisted gold nanoparticle aggregates for electrochemical signal amplification of lipopolysaccharide sensing. Biosens. Bioelectron. 126, 529–534 (2019). https://doi.org/10.1016/j.bios.2018.11.021
J.H. Kim, J.S. Suh, J. Yang, Labeling-free detection of ECD-HER2 protein using aptamer-based nano-plasmonic sensor. Nanotechnology 31(17), 175501 (2020). https://doi.org/10.1088/1361-6528/ab68fa
V.A. Tran, V.G. Vo, K. Shim, S.W. Lee, S.S.A. An, Multimodal mesoporous silica nanocarriers for dual stimuli-responsive drug release and excellent photothermal ablation of cancer cells. Int. J. Nanomed. 15(1), 7667–7685 (2020). https://doi.org/10.2147/IJN.S254344
Y. Zhang, C. Guo, L. Liu, J. Xu, H. Jiang et al., Zno-based multifunctional nanocomposites to inhibit progression and metastasis of melanoma by eliciting antitumor immunity via immunogenic cell death. Theranostics 10(24), 11197–11214 (2020). https://doi.org/10.7150/thno.44920
D. Jiang, D. Ni, Z.T. Rosenkrans, P. Huang, X. Yan et al., Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 48(14), 3683–3704 (2019). https://doi.org/10.1039/c8cs00718g
S. Gao, H. Lin, H. Zhang, H. Yao, Y. Chen et al., Nanocatalytic tumor therapy by biomimetic dual inorganic nanozyme-catalyzed cascade reaction. Adv. Sci. 6(3), 1801733 (2019). https://doi.org/10.1002/advs.201801733
X. Hu, F. Li, F. Xia, X. Guo, N. Wang et al., Biodegradation-mediated enzymatic activity-tunable molybdenum oxide nanourchins for tumor-specific cascade catalytic therapy. J. Am. Chem. Soc. 142(3), 1636–1644 (2020). https://doi.org/10.1021/jacs.9b13586
Y.F. Liu, Y. Cheng, H. Zhang, M. Zhou, Y.J. Yu et al., Integrated cascade nanozyme catalyzes in vivo ROS scavenging for anti-inflammatory therapy. Sci. Adv. 6(1), 2695 (2020). https://doi.org/10.1126/sciadv.abb2695
N. Kamaly, B. Yameen, J. Wu, O.C. Farokhzad, Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem. Rev. 116(4), 2602–2663 (2016). https://doi.org/10.1021/acs.chemrev.5b00346
K. Ulbrich, K. Hola, V. Subr, A. Bakandritsos, J. Tucek et al., Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 116(9), 5338–5431 (2016). https://doi.org/10.1021/acs.chemrev.5b00589
I. Ekladious, Y.L. Colson, M.W. Grinstaff, Polymer-drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 18(4), 273–294 (2019). https://doi.org/10.1038/s41573-018-0005-0
S. Rashki, K. Asgarpour, H. Tarrahimofrad, M. Hashemipour, M.S. Ebrahimi et al., Chitosan-based nanoparticles against bacterial infections. Carbohyd. Polym. 251(1), 117108 (2021). https://doi.org/10.1016/j.carbpol.2020.117108
M. Lara-Velazquez, R. Alkharboosh, E.S. Norton, C. Ramirez-Loera, W.D. Freeman et al., Chitosan-based non-viral gene and drug delivery systems for brain cancer. Front. Neurol. 11(1), 740 (2020). https://doi.org/10.3389/fneur.2020.00740
X. Lang, T. Wang, M. Sun, X. Chen, Y. Liu, Advances and applications of chitosan-based nanomaterials as oral delivery carriers: a review. Int. J. Biol. Macromol. 154(1), 433–445 (2020). https://doi.org/10.1016/j.ijbiomac.2020.03.148
P. Severino, C.F. da Silva, L.N. Andrade, D. de Lima-Oliveira, J. Campos et al., Alginate nanoparticles for drug delivery and targeting. Curr. Pharm. Des. 25(11), 1312–1334 (2019). https://doi.org/10.2174/1381612825666190425163424
J. Ding, Y. Yao, J. Li, Y. Duan, J.R. Nakkala et al., A reactive oxygen species scavenging and O2 generating injectable hydrogel for myocardial infarction treatment in vivo. Small 16, 2005038 (2020). https://doi.org/10.1002/smll.202005038
Y. Hong, F. Zhou, Y. Hua, X. Zhang, C. Ni et al., A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 10(1), 2060 (2019). https://doi.org/10.1038/s41467-019-10004-7
Y.W. Chen, W.L. Shen, C.Q. Tang, J.Y. Huang, C.M. Fan et al., Targeted pathological collagen delivery of sustained-release rapamycin to prevent heterotopic ossification. Sci. Adv. 6(1), 9526 (2019). https://doi.org/10.1126/sciadv.aay9526
J. Di, F. Xie, Y. Xu, When liposomes met antibodies: drug delivery and beyond. Adv. Drug Deliver. Rev. (2020). https://doi.org/10.1016/j.addr.2020.09.003
J.J. Sonju, A. Dahal, S.S. Singh, S.D. Jois, Peptide-functionalized liposomes as therapeutic and diagnostic tools for cancer treatment. J. Control. Release (2020). https://doi.org/10.1016/j.jconrel.2020.09.055
A. Al-Saqr, M.F. Aldawsari, H. Alrbyawi, I. Poudel, M. Annaji et al., Co-delivery of hispolon and doxorubicin liposomes improves efficacy against melanoma cells. AAPS PharmSciTech 21(8), 304 (2020). https://doi.org/10.1208/s12249-020-01846-2
Q. Wang, P. Tardi, N. Sadowski, S. Xie, D. Heller et al., Pharmacokinetics, drug metabolism, and tissue distribution of CPX-351 in animals. Nanomed. Nanotechnol. 30(1), 102275 (2020). https://doi.org/10.1016/j.nano.2020.102275
L. Shi, Y. Wang, Q. Wang, Z. Jiang, L. Ren et al., Transforming a toxic drug into an efficacious nanomedicine using a lipoprodrug strategy for the treatment of patient-derived melanoma xenografts. J. Control. Release 324(1), 289–302 (2020). https://doi.org/10.1016/j.jconrel.2020.05.025
Y. Tang, X. Wang, J. Li, Y. Nie, G. Liao et al., Overcoming the reticuloendothelial system barrier to drug delivery with a “don’t-eat-us” strategy. ACS Nano 13(11), 13015–13026 (2019). https://doi.org/10.1021/acsnano.9b05679
Z. Zhang, J. Guan, Z. Jiang, Y. Yang, J. Liu et al., Brain-targeted drug delivery by manipulating protein corona functions. Nat. Commun. 10(1), 3561 (2019). https://doi.org/10.1038/s41467-019-11593-z
H. Wang, X. Xu, X. Guan, S. Shen, X. Huang et al., Liposomal 9-aminoacridine for treatment of ischemic stroke: from drug discovery to drug delivery. Nano Lett. 20(3), 1542–1551 (2020). https://doi.org/10.1021/acs.nanolett.9b04018
J. Zhou, A.V. Kroll, M. Holay, R.H. Fang, L. Zhang, Biomimetic nanotechnology toward personalized vaccines. Adv. Mater. 32(13), e1901255 (2020). https://doi.org/10.1002/adma.201901255
Z. Chen, Z. Wang, Z. Gu, Bioinspired and biomimetic nanomedicines. Acc. Chem. Res. 52(5), 1255–1264 (2019). https://doi.org/10.1021/acs.accounts.9b00079
R.H. Fang, A.V. Kroll, W. Gao, L. Zhang, Cell membrane coating nanotechnology. Adv. Mater. 30(23), e1706759 (2018). https://doi.org/10.1002/adma.201706759
J.C. Harris, M.A. Scully, E.S. Day, Cancer cell membrane-coated nanoparticles for cancer management. Cancers 11(12), 1836 (2019). https://doi.org/10.3390/cancers11121836
V. Cagno, P. Andreozzi, M. D’Alicarnasso, P. Jacob-Silva, M. Mueller et al., Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 17(2), 195–203 (2018). https://doi.org/10.1038/nmat5053
J. Wang, P. Li, Y. Yu, Y. Fu, H. Jiang et al., Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 367(6480), 0810 (2020). https://doi.org/10.1126/science.aau0810
Y. Liu, J. Luo, X. Chen, W. Liu, T. Chen, Cell membrane coating technology: a promising strategy for biomedical applications. Nano-Micro Lett. 11(1), 100 (2019). https://doi.org/10.1007/s40820-019-0330-9
R. Molinaro, C. Corbo, J.O. Martinez, F. Taraballi, M. Evangelopoulos et al., Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 15(9), 1037–1046 (2016). https://doi.org/10.1038/nmat4644
J. Koo, T. Escajadillo, L. Zhang, V. Nizet, S.M. Lawrence, Erythrocyte-coated nanoparticles block cytotoxic effects of group b streptococcus beta-hemolysin/cytolysin. Front. Pediatr. 7(1), 410 (2019). https://doi.org/10.3389/fped.2019.00410
Y. Lu, Q. Hu, C. Jiang, Z. Gu, Platelet for drug delivery. Curr. Opin. Biotechnol. 58(1), 81–91 (2019). https://doi.org/10.1016/j.copbio.2018.11.010
J. Zhuang, H. Gong, J.R. Zhou, Q.Z. Zhang, W.W. Gao et al., Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles. Sci. Adv. 6(1), 6108 (2020). https://doi.org/10.1126/sciadv.aaz6108
G. Zhang, G.R. Campbell, Q.Z. Zhang, E. Maule, J. Hanna et al., Cd4+ t cell-mimicking nanoparticles broadly neutralize HIV-1 and suppress viral replication through autophagy. mBio 11(5), 00903–00920 (2020). https://doi.org/10.1128/mBio.00903-20
C. Tapeinos, F. Tomatis, M. Battaglini, A. Larranaga, A. Marino et al., Cell membrane-coated magnetic nanocubes with a homotypic targeting ability increase intracellular temperature due to ros scavenging and act as a versatile theranostic system for glioblastoma multiforme. Adv. Health Mater. 8(18), e1900612 (2019). https://doi.org/10.1002/adhm.201900612
Y. Jiang, N. Krishnan, J. Zhou, S. Chekuri, X. Wei et al., Engineered cell-membrane-coated nanoparticles directly present tumor antigens to promote anticancer immunity. Adv. Mater. 32(30), e2001808 (2020). https://doi.org/10.1002/adma.202001808
Y. Zhang, Y. Chen, C. Lo, J. Zhuang, P. Angsantikul et al., Inhibition of pathogen adhesion by bacterial outer membrane-coated nanoparticles. Angew. Chem. Int. Ed. 58(33), 11404–11408 (2019). https://doi.org/10.1002/anie.201906280
Q. Zhang, A. Honko, J. Zhou, H. Gong, S.N. Downs et al., Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett. 20(7), 5570–5574 (2020). https://doi.org/10.1021/acs.nanolett.0c02278
F. Gorjikhah, S. Davaran, R. Salehi, M. Bakhtiari, A. Hasanzadeh et al., Improving “lab-on-a-chip” techniques using biomedical nanotechnology: a review. Artif. Cell. Nanomed. Biotechnol. 44(7), 1609–1614 (2016). https://doi.org/10.3109/21691401.2015.1129619
B. Hu, C. Owh, P.L. Chee, W.R. Leow, X. Liu et al., Supramolecular hydrogels for antimicrobial therapy. Chem. Soc. Rev. 47(18), 6917–6929 (2018). https://doi.org/10.1039/c8cs00128f
S. Wang, Y. Gao, Q. Jin, J. Ji, Emerging antibacterial nanomedicine for enhanced antibiotic therapy. Biomater. Sci. 8, 6825–6839 (2020). https://doi.org/10.1039/d0bm00974a
D.G. Remick, Cytokine therapeutics for the treatment of sepsis: why has nothing worked? Curr. Pharm. Des. 9(1), 75–82 (2003). https://doi.org/10.2174/1381612033392567
M.D.V. Marco-Ranieri, M.D.B. Taylor-Thompson, M.D. Philip, S. Barie, M.D. Jean-François-Dhainaut et al., Drotrecogin alfa (activated) in adults with septic shock. N. Engl. J. Med. 366(22), 2055–2064 (2012). https://doi.org/10.1056/NEJMoa1202290
J. Lou-Franco, B. Das, C. Elliott, C. Cao, Gold nanozymes: from concept to biomedical applications. Nano-Micro Lett. 13(1), 10 (2020). https://doi.org/10.1007/s40820-020-00532-z
N. Feliu, D. Docter, M. Heine, P. Del Pino, S. Ashraf et al., In vivo degeneration and the fate of inorganic nanoparticles. Chem. Soc. Rev. 45(9), 2440–2457 (2016). https://doi.org/10.1039/c5cs00699f
N.D. Donahue, H. Acar, S. Wilhelm, Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliver. Rev. 143(1), 68–96 (2019). https://doi.org/10.1016/j.addr.2019.04.008
S. Ravindran, J.K. Suthar, R. Rokade, P. Deshpande, P. Singh et al., Pharmacokinetics, metabolism, distribution and permeability of nanomedicine. Curr. Drug Metab. 19(4), 327–334 (2018). https://doi.org/10.2174/1389200219666180305154119
S.E. McNeil, Nanoparticle therapeutics a personal perspective. Wires Nanomed. Nanobi. 1(1), 264–271 (2009). https://doi.org/10.1002/wnan.006
S. Ranjbar-Bahadori, A. Mulgaonkar, R. Hart, C.Y. Wu, D. Zhang et al., Radiolabeling strategies and pharmacokinetic studies for metal based nanotheranostics. Wires Nanomed. Nanobi. (2020). https://doi.org/10.1002/wnan.1671
H. Arami, A. Khandhar, D. Liggitt, K.M. Krishnan, In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 44(23), 8576–8607 (2015). https://doi.org/10.1039/c5cs00541h
J. Lu, J. Wang, D. Ling, Surface engineering of nanoparticles for targeted delivery to hepatocellular carcinoma. Small 14(5), 1702037 (2018). https://doi.org/10.1002/smll.201702037
E. Ben-Akiva, R.A. Meyer, H.Z. Yu, J.T. Smith, D.M. Pardoll et al., Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Sci. Adv. 6(1), 9035 (2020). https://doi.org/10.1126/sciadv.aay9035
F. Bloos, K. Reinhart, Rapid diagnosis of sepsis. Virulence 5(1), 154–160 (2014). https://doi.org/10.4161/viru.27393
X.Z. Mou, X.Y. Chen, J. Wang, Z. Zhang, Y. Yang et al., Bacteria-instructed click chemistry between functionalized gold nanoparticles for point-of-care microbial detection. ACS Appl. Mater. Interfaces 11(26), 23093–23101 (2019). https://doi.org/10.1021/acsami.9b09279
M. Sun, A. Qu, C. Hao, X. Wu, L. Xu et al., Chiral upconversion heterodimers for quantitative analysis and bioimaging of antibiotic-resistant bacteria in vivo. Adv. Mater. 30(50), e1804241 (2018). https://doi.org/10.1002/adma.201804241
I.K. Herrmann, S. Bertazzo, D.J.P. O’Callaghan, A.A. Schlegel et al., Differentiating sepsis from non-infectious systemic inflammation based on microvesicle-bacteria aggregation. Nanoscale 7(32), 13511–13520 (2015). https://doi.org/10.1039/c5nr01851j
L. Wang, W.J. Zhao, M.B. O’Donoghue, W. Tan, Fluorescent nanoparticles for multiplexed bacteria monitoring. Bioconjugate Chem. 18(2), 297–301 (2007). https://doi.org/10.1021/bc060255n
G. Santopolo, A. Doménech-Sánchez, S.M. Russell, R. de la Rica, Ultrafast and ultrasensitive naked-eye detection of urease-positive bacteria with plasmonic nanosensors. ACS Sensors 4(4), 961–967 (2019). https://doi.org/10.1021/acssensors.9b00063
C.-W. Lee, H.-Y. Chang, J.-K. Wu, F.-G. Tseng, Ultra-sensitive electrochemical detection of bacteremia enabled by redox-active gold nanoparticles (raGNPs) in a nano-sieving microfluidic system (NS-MFS). Biosens. Bioelectron. 133(1), 215–222 (2019). https://doi.org/10.1016/j.bios.2019.03.040
J. Gao, L. Li, P.L. Ho, G.C. Mak, H. Gu et al., Combining fluorescent probes and biofunctional magnetic nanoparticles for rapid detection of bacteria in human blood. Adv. Mater. 18(23), 3145–3148 (2006). https://doi.org/10.1002/adma.200601058
A. Belushkin, F. Yesilkoy, H. Altug, Nanoparticle-enhanced plasmonic biosensor for digital biomarker detection in a microarray. ACS Nano 12(5), 4453–4461 (2018). https://doi.org/10.1021/acsnano.8b00519
D.K. Changlin-Fu, Pilot application of magnetic nanoparticle-based biosensor for necrotizing enterocolitis. J. Proteomics. Bioinf. 5(1), 002 (2015). https://doi.org/10.4172/jpb.S5-002
R.K. Gupta, A. Periyakaruppan, M. Meyyappan, J.E. Koehne, Label-free detection of c-reactive protein using a carbon nanofiber based biosensor. Biosens. Bioelectron. 59(1), 112–119 (2014). https://doi.org/10.1016/j.bios.2014.03.027
L. Cao, J. Kiely, M. Piano, R. Luxton, A copper oxide/zinc oxide composite nano-surface for use in a biosensor. Materials 12(7), 1126 (2019). https://doi.org/10.3390/ma12071126
L. Zhang, S. Tong, J. Zhou, G. Bao, Accurate quantification of disease markers in human serum using iron oxide nanoparticle-linked immunosorbent assay. Theranostics 6(9), 1353–1361 (2016). https://doi.org/10.7150/thno.16093
L. Cao, J. Kiely, M. Piano, R. Luxton, Facile and inexpensive fabrication of zinc oxide based bio-surfaces for c-reactive protein detection. Sci. Rep. 8(1), 12687 (2018). https://doi.org/10.1038/s41598-018-30793-z
K.M. Park, D.J. Chung, M. Choi, T. Kang, J. Jeong, Fluorescent fullerene nanoparticle-based lateral flow immunochromatographic assay for rapid quantitative detection of c-reactive protein. Nano Converg. 6(1), 35 (2019). https://doi.org/10.1186/s40580-019-0207-0
I. Letchumanan, M.K. Md-Arshad, S.R. Balakrishnan, S.C.B. Gopinath, Gold-nanorod enhances dielectric voltammetry detection of c-reactive protein: a predictive strategy for cardiac failure. Biosens. Bioelectron. 130(1), 40–47 (2019). https://doi.org/10.1016/j.bios.2019.01.042
A. Prajapati, N. Verma, A. Pandya, Highly sensitive vertical flow based point-of-care immunokit for rapid and early detection of human CRP as a cardiovascular risk factor. Biomed. Microdevices 22(2), 28 (2020). https://doi.org/10.1007/s10544-020-00480-w
A. Liu, X. Wang, Amperometric immunosensor of procalcitonin based on amplification strategy of ferrocene-modified gold nanoparticles. Int. J. Electrochem. Sci. 10(1), 9342–9350 (2015)
P. Li, W. Zhang, X. Zhou, L. Zhang, C60 carboxyfullerene-based functionalised nanohybrids as signal-amplifying tags for the ultrasensitive electrochemical detection of procalcitonin. Clin. Biochem. 48(3), 156–161 (2015). https://doi.org/10.1016/j.clinbiochem.2014.09.017
Z.H. Yang, S. Ren, Y. Zhuo, R. Yuan, Y.Q. Chai, Cu/Mn double-doped CeO2 nanocomposites as signal tags and signal amplifiers for sensitive electrochemical detection of procalcitonin. Anal. Chem. 89(24), 13349–13356 (2017). https://doi.org/10.1021/acs.analchem.7b03502
C.Y. Chiang, T.T. Huang, C.H. Wang, C.J. Huang, T.H. Tsai et al., Fiber optic nanogold-linked immunosorbent assay for rapid detection of procalcitonin at femtomolar concentration level. Biosens. Bioelectron. 151(1), 111871 (2020). https://doi.org/10.1016/j.bios.2019.111871
F. Liu, G. Xiang, R. Yuan, X. Chen, F. Luo et al., Procalcitonin sensitive detection based on graphene-gold nanocomposite film sensor platform and single-walled carbon nanohorns/hollow pt chains complex as signal tags. Biosens. Bioelectron. 60(1), 210–217 (2014). https://doi.org/10.1016/j.bios.2014.03.071
W. Jing, Y. Wang, Y. Yang, Y. Wang, G. Ma et al., Time-resolved digital immunoassay for rapid and sensitive quantitation of procalcitonin with plasmonic imaging. ACS Nano 13(8), 8609–8617 (2019). https://doi.org/10.1021/acsnano.9b02771
X. Xu, X. Song, R. Nie, Y. Yang, Y. Chen et al., Ultra-sensitive capillary immunosensor combining porous-layer surface modification and biotin-streptavidin nano-complex signal amplification: application for sensing of procalcitonin in serum. Talanta 205(1), 120089 (2019). https://doi.org/10.1016/j.talanta.2019.06.089
Q. Lin, J. Wu, X. Fang, J. Kong, Washing-free centrifugal microchip fluorescence immunoassay for rapid and point-of-care detection of protein. Anal. Chim. Acta 1118(1), 18–25 (2020). https://doi.org/10.1016/j.aca.2020.04.031
W.W. Xiong, G.H. Yang, X.C. Wu, J.J. Zhu, Aqueous synthesis of color-tunable CuInS2/ZnS nanocrystals for the detection of human interleukin 6. ACS Appl. Mater. Interfaces 5(16), 8210–8216 (2013). https://doi.org/10.1021/am402328t
Z. Hao, Y. Pan, W. Shao, Q. Lin, X. Zhao, Graphene-based fully integrated portable nanosensing system for on-line detection of cytokine biomarkers in saliva. Biosens. Bioelectron. 134(1), 16–23 (2019). https://doi.org/10.1016/j.bios.2019.03.053
U.Y. Lau, S.S. Saxer, J. Lee, E. Bat, H.D. Maynard, Direct write protein patterns for multiplexed cytokine detection from live cells using electron beam lithography. ACS Nano 10(1), 723–729 (2016). https://doi.org/10.1021/acsnano.5b05781
E. Sánchez-Tirado, C. Salvo, A. González-Cortés, P. Yáñez-Sedeño, F. Langa et al., Electrochemical immunosensor for simultaneous determination of interleukin-1 beta and tumor necrosis factor alpha in serum and saliva using dual screen printed electrodes modified with functionalized double–walled carbon nanotubes. Anal. Chim. Acta 959(1), 66–73 (2017). https://doi.org/10.1016/j.aca.2016.12.034
B.R. Oh, N.T. Huang, W.Q. Chen, J. Hwan-Seo, P.Y. Chen et al., Integrated nanoplasmonic sensing for cellular functional immunoanalysis using human blood. ACS Nano 8(3), 2667–2676 (2014). https://doi.org/10.1021/nn406370u
Y. Park, B. Ryu, B.R. Oh, Y. Song, X. Liang et al., Biotunable nanoplasmonic filter on few-layer MoS2 for rapid and highly sensitive cytokine optoelectronic immunosensing. ACS Nano 11(6), 5697–5705 (2017). https://doi.org/10.1021/acsnano.7b01162
J. Min, M. Nothing, B. Coble, H. Zheng, J. Park et al., Integrated biosensor for rapid and point-of-care sepsis diagnosis. ACS Nano 12(4), 3378–3384 (2018). https://doi.org/10.1021/acsnano.7b08965
P.Y. Chen, M.T. Chung, W. McHugh, R. Nidetz, Y.W. Li et al., Multiplex serum cytokine immunoassay using nanoplasmonic biosensor microarrays. ACS Nano 9(4), 4173–4181 (2015). https://doi.org/10.1021/acsnano.5b00396
M.N. Hsu, S.C. Wei, D.T. Phan, Y. Zhang, C.H. Chen, Nano-in-micro smart hydrogel composite for a rapid sensitive immunoassay. Adv. Healthc. Mater. 8(4), e1801277 (2019). https://doi.org/10.1002/adhm.201801277
H.W. Gu, P.L. Ho, K.W.T. Tsang, L. Wang, B. Xu, Using biofunctional magnetic nanoparticles to capture vancomycin-resistant. J. Am. Chem. Soc. 125, 15702–15703 (2003). https://doi.org/10.1021/ja0359310
R. Frank, R. Hargreaves, Clinical biomarkers in drug discovery and development. Nat. Rev. Drug Discov. 2(7), 566–580 (2003). https://doi.org/10.1038/nrd1130
C.F. Oliveira, F.A. Botoni, C.R. Oliveira, C.B. Silva, H.A. Pereira et al., Procalcitonin versus c-reactive protein for guiding antibiotic therapy in sepsis: a randomized trial. Crit. Care Med. 41(10), 2336–2343 (2013). https://doi.org/10.1097/CCM.0b013e31828e969f
M.K. Masud, J. Na, M. Younus, M.S.A. Hossain, Y. Bando et al., Superparamagnetic nanoarchitectures for disease-specific biomarker detection. Chem. Soc. Rev. 48(24), 5717–5751 (2019). https://doi.org/10.1039/c9cs00174c
M. Perfezou, A. Turner, A. Merkoci, Cancer detection using nanoparticle-based sensors. Chem. Soc. Rev. 41(7), 2606–2622 (2012). https://doi.org/10.1039/c1cs15134g
K.K. Jain, Applications of nanobiotechnology in clinical diagnostics. Clin. Chem. 53(11), 2002–2009 (2007). https://doi.org/10.1373/clinchem.2007.090795
N. Widiarti, J.K. Sae, S. Wahyuni, Synthesis CuO–ZnO nanocomposite and its application as an antibacterial agent. IOP Conf. Ser. Mater. Sci. Eng. 172(1), 012036 (2017). https://doi.org/10.1088/1757-899x/172/1/012036
P. Schuetz, C. Bretscher, L. Bernasconi, B. Mueller, Overview of procalcitonin assays and procalcitonin-guided protocols for the management of patients with infections and sepsis. Expert Rev. Mol. Diagn. 17(6), 593–601 (2017). https://doi.org/10.1080/14737159.2017.1324299
C. Wacker, A. Prkno, F.M. Brunkhorst, P. Schlattmann, Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect. Dis. 13(5), 426–435 (2013). https://doi.org/10.1016/s1473-3099(12)70323-7
M. Stocker, W. van Herk, S. el Helou, S. Dutta, M.S. Fontana et al., Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: a multicentre, randomised controlled trial (neopins). Lancet 390(10097), 871–881 (2017). https://doi.org/10.1016/s0140-6736(17)31444-7
M. Meisner, Update on procalcitonin measurements. Ann. Lab. Med. 34(4), 263–273 (2014). https://doi.org/10.3343/alm.2014.34.4.263
E. Mauriz, P. Dey, L.M. Lechuga, Advances in nanoplasmonic biosensors for clinical applications. The Analyst 144(24), 7105–7129 (2019). https://doi.org/10.1039/c9an00701f
C. Nedeva, J. Menassa, H. Puthalakath, Sepsis: inflammation is a necessary evil. Front. Cell Dev. Biol. 7(1), 108 (2019). https://doi.org/10.3389/fcell.2019.00108
D. Rittirsch, M.A. Flierl, P.A. Ward, Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 8(10), 776–787 (2008). https://doi.org/10.1038/nri2402
J.D. Spitzberg, A. Zrehen, X.F. van Kooten, A. Meller, Plasmonic-nanopore biosensors for superior single-molecule detection. Adv. Mater. 31(23), e1900422 (2019). https://doi.org/10.1002/adma.201900422
Y.C. Yeh, T.H. Huang, S.C. Yang, C.C. Chen, J.Y. Fang, Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances. Front. Chem. 8(1), 286 (2020). https://doi.org/10.3389/fchem.2020.00286
M.A. Mohamed, Myco-engineered gold nanoparticles from jahnula aquatica coated with ampicillin/amoxicillin and their antibacterial and anticancer activity against cancer cells. Biotechnol. Lett. 42(1), 151–170 (2020). https://doi.org/10.1007/s10529-019-02764-5
G.B. Hwang, H. Huang, G. Wu, J. Shin, A. Kafizas et al., Photobactericidal activity activated by thiolated gold nanoclusters at low flux levels of white light. Nat. Commun. 11(1), 1207 (2020). https://doi.org/10.1038/s41467-020-15004-6
D.P. Linklater, V.A. Baulin, X. Le Guevel, J.B. Fleury, E. Hanssen et al., Antibacterial action of nanoparticles by lethal stretching of bacterial cell membranes. Adv. Mater. 32, 2005679 (2020). https://doi.org/10.1002/adma.202005679
M.A.M. Jahromi, P.S. Zangabad, S.M.M. Basri, K.S. Zangabad, A. Ghamarypour et al., Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliver. Rev. 123(1), 33–64 (2018). https://doi.org/10.1016/j.addr.2017.08.001
J. Verma, J. Kanoujia, P. Parashar, C.B. Tripathi, S.A. Saraf, Wound healing applications of sericin/chitosan-capped silver nanoparticles incorporated hydrogel. Drug Deliv. Transl. Res. 7(1), 77–88 (2017). https://doi.org/10.1007/s13346-016-0322-y
L. Rizzello, P.P. Pompa, Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 43(5), 1501–1518 (2014). https://doi.org/10.1039/c3cs60218d
X. Li, H. Bai, Y. Yang, J. Yoon, S. Wang et al., Supramolecular antibacterial materials for combatting antibiotic resistance. Adv. Mater. 31(5), e1805092 (2019). https://doi.org/10.1002/adma.201805092
I.R. Scolari, P.L. Paez, M.M. Musri, J.P. Petiti, A. Torres et al., Rifampicin loaded in alginate/chitosan nanoparticles as a promising pulmonary carrier against staphylococcus aureus. Drug Deliv. Transl. Res. 10(5), 1403–1417 (2020). https://doi.org/10.1007/s13346-019-00705-3
D. Mazzaccaro, R. Ticozzi, S. D’Alessandro, S. Delbue, G. Nano et al., Effect of antibiotic-loaded chitosan nanodroplets on enterococci isolated from chronic ulcers of the lower limbs. Future Microbiol. 15(13), 1227–1236 (2020). https://doi.org/10.2217/fmb-2019-0255
C. Hu, F. Zhang, L. Long, Q. Kong, R. Luo et al., Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control Release 324(1), 204–217 (2020). https://doi.org/10.1016/j.jconrel.2020.05.010
L.D. Duceac, G. Calin, L. Eva, C. Marcu, E.R. Bogdan-Goroftei et al., Third-generation cephalosporin-loaded chitosan used to limit microorganisms resistance. Materials 13(21), 4792 (2020). https://doi.org/10.3390/ma13214792
Z. Li, G. Luo, W.P. Hu, J.L. Hua, S. Geng et al., Mediated drug release from nanovehicles by black phosphorus quantum dots for efficient therapy of chronic obstructive pulmonary disease. Angew. Chem. Int. Ed. 59(1), 20568–20576 (2020). https://doi.org/10.1002/anie.202008379
E.B. Yahya, F. Jummaat, A.A. Amirul, A.S. Adnan, N.G. Olaiya et al., A review on revolutionary natural biopolymer-based aerogels for antibacterial delivery. Antibiotics 9(10), 648 (2020). https://doi.org/10.3390/antibiotics9100648
X.X. Wu, Y. Zhang, T. Hu, W.X. Li, Z.L. Li et al., Long-term antibacterial composite via alginate aerogel sustained release of antibiotics and Cu used for bone tissue bacteria infection. Int. J. Biol. Macromol. (2020). https://doi.org/10.1016/j.ijbiomac.2020.11.075
S. Obuobi, K. Julin, E.G.A. Fredheim, M. Johannessen, N. Skalko-Basnet, Liposomal delivery of antibiotic loaded nucleic acid nanogels with enhanced drug loading and synergistic anti-inflammatory activity against S. aureus intracellular infections. J. Control. Release 324(1), 620–632 (2020). https://doi.org/10.1016/j.jconrel.2020.06.002
E. Teirlinck, A. Barras, J. Liu, J.C. Fraire, T. Lajunen et al., Exploring light-sensitive nanocarriers for simultaneous triggered antibiotic release and disruption of biofilms upon generation of laser-induced vapor nanobubbles. Pharmaceutics 11(5), 201 (2019). https://doi.org/10.3390/pharmaceutics11050201
K.A. Vandera, P. Picconi, M. Valero, G. Gonzalez-Gaitano, A. Woods et al., Antibiotic-in-cyclodextrin-in-liposomes: formulation development and interactions with model bacterial membranes. Mol. Pharmaceut. 17(7), 2354–2369 (2020). https://doi.org/10.1021/acs.molpharmaceut.0c00096
X. Pang, Q. Xiao, Y. Cheng, E. Ren, L. Lian et al., Bacteria-responsive nanoliposomes as smart sonotheranostics for multidrug resistant bacterial infections. ACS Nano 13(2), 2427–2438 (2019). https://doi.org/10.1021/acsnano.8b09336
Y. Wu, Z. Song, H. Wang, H. Han, Endogenous stimulus-powered antibiotic release from nanoreactors for a combination therapy of bacterial infections. Nat. Commun. 10(1), 4464 (2019). https://doi.org/10.1038/s41467-019-12233-2
G. Yang, S. Chen, J. Zhang, Bioinspired and biomimetic nanotherapies for the treatment of infectious diseases. Front. Pharmacol. 10(1), 751 (2019). https://doi.org/10.3389/fphar.2019.00751
W. Gao, L. Zhang, Coating nanoparticles with cell membranes for targeted drug delivery. J. Drug Target. 23(7–8), 619–626 (2015). https://doi.org/10.3109/1061186X.2015.1052074
W. Huang, Q. Zhang, W. Li, M. Yuan, J. Zhou et al., Development of novel nanoantibiotics using an outer membrane vesicle-based drug efflux mechanism. J. Control. Release 317(1), 1–22 (2020). https://doi.org/10.1016/j.jconrel.2019.11.017
F. Gao, L. Xu, B. Yang, F. Fan, L. Yang, Kill the real with the fake: Eliminate intracellular staphylococcus aureus using nanoparticle coated with its extracellular vesicle membrane as active-targeting drug carrier. ACS Infect. Dis. 5(2), 218–227 (2019). https://doi.org/10.1021/acsinfecdis.8b00212
R. Pascale, M. Giannella, M. Bartoletti, P. Viale, F. Pea, Use of meropenem in treating carbapenem-resistant enterobacteriaceae infections. Expert Rev. Anti-Infe. Therapy 17(10), 819–827 (2019). https://doi.org/10.1080/14787210.2019.1673731
F.M. Brunkhorst, M. Oppert, G. Marx, F. Bloos, K. Ludewig et al., Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA 307(22), 2390–2399 (2012). https://doi.org/10.1001/jama.2012.5833
J.A. Roberts, M.H. Abdul-Aziz, J.S. Davis, J.M. Dulhunty, M.O. Cotta et al., Continuous versus intermittent b-lactam infusion in severe sepsis: a meta-analysis of individual patient data from randomized trials. Am. J. Resp. Crit. Care 194(6), 681–691 (2016). https://doi.org/10.1164/rccm.201601-0024OC
P.N.A. Harris, P.A. Tambyah, D.C. Lye, Y. Mo, T.H. Lee et al., N. the Australasian Society for Infectious Disease Clinical Research. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with e coli or klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. Jama 320(10), 984–994 (2018). https://doi.org/10.1001/jama.2018.12163
A. Abdelkader, M.A. El-Mokhtar, O. Abdelkader, M.A. Hamad, M. Elsabahy et al., Ultrahigh antibacterial efficacy of meropenem-loaded chitosan nanoparticles in a septic animal model. Carbohyd. Polym. 174(1), 1041–1050 (2017). https://doi.org/10.1016/j.carbpol.2017.07.030
J. Ndayishimiye, A. Popat, M. Blaskovich, J.R. Falconer, Formulation technologies and advances for oral delivery of novel nitroimidazoles and antimicrobial peptides. J. Control. Release (2020). https://doi.org/10.1016/j.jconrel.2020.05.002
A.C. Saude, A.S. Ombredane, O.N. Silva, J.A. Barbosa, S.E. Moreno et al., Clavanin bacterial sepsis control using a novel methacrylate nanocarrier. Int. J. Nanomed. 9(1), 5055–5069 (2014). https://doi.org/10.2147/IJN.S66300
C. Falciani, F. Zevolini, J. Brunetti, G. Riolo, R. Gracia et al., Antimicrobial peptide-loaded nanoparticles as inhalation therapy for pseudomonas aeruginosa infections. Int. J. Nanomed. 15(1), 1117–1128 (2020). https://doi.org/10.2147/IJN.S218966
N. Mookherjee, M.A. Anderson, H.P. Haagsman, D.J. Davidson, Antimicrobial host defence peptides: functions and clinical potential. Nat. Rev. Drug Discov. 19(5), 311–332 (2020). https://doi.org/10.1038/s41573-019-0058-8
A.J. Beevers, A.M. Dixon, Helical membrane peptides to modulate cell function. Chem. Soc. Rev. 39(6), 2146–2157 (2010). https://doi.org/10.1039/b912944h
T. Ganz, Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3(9), 710–720 (2003). https://doi.org/10.1038/nri1180
B.P. Lazzaro, M. Zasloff, J. Rolff, Antimicrobial peptides: application informed by evolution. Science 368(6490), 5480 (2020). https://doi.org/10.1126/science.aau5480
Y. Scindia, E. Wlazlo, J. Leeds, V. Loi, J. Ledesma et al., Protective role of hepcidin in polymicrobial sepsis and acute kidney injury. Front. Pharm. 10(1), 615 (2019). https://doi.org/10.3389/fphar.2019.00615
Y.H. Huang, Y.L. Yang, M.M. Tiao, H.C. Kuo, L.T. Huang et al., Hepcidin protects against lipopolysaccharide-induced liver injury in a mouse model of obstructive jaundice. Peptides 35(2), 212–217 (2012). https://doi.org/10.1016/j.peptides.2012.03.032
N.H. Salzman, D. Ghosh, K.M. Huttner, Y. Patersonk, C.L. Bevins, Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422(6931), 522–526 (2003). https://doi.org/10.1038/nature01520
E. de Leeuw, S.R. Burks, X. Li, J.P. Kao, W. Lu, Structure-dependent functional properties of human defensin 5. FEBS Lett. 581(3), 515–520 (2007). https://doi.org/10.1016/j.febslet.2006.12.036
C. Wang, M. Shen, N. Gohain, W.D. Tolbert, F. Chen et al., Design of a potent antibiotic peptide based on the active region of human defensin 5. J. Med. Chem. 58(7), 3083–3093 (2015). https://doi.org/10.1021/jm501824a
A. de Breij, M. Riool, R.A. Cordfunke, N. Malanovic, L. de Boer et al., The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 10, 4044 (2018). https://doi.org/10.1126/scitranslmed.aan4044
L. Liu, K. Xu, H. Wang, P.K. Tan, W. Fan et al., Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat. Nanotechnol. 4(7), 457–463 (2009). https://doi.org/10.1038/nnano.2009.153
B. Mathew, R. Nagaraj, Antimicrobial activity of human alpha-defensin 5 and its linear analogs: N-terminal fatty acylation results in enhanced antimicrobial activity of the linear analogs. Peptides 71, 128–140 (2015). https://doi.org/10.1016/j.peptides.2015.07.009
J.J. Lee, K.J. Jeong, M. Hashimoto, A.H. Kwon, A. Rwei et al., Synthetic ligand-coated magnetic nanoparticles for microfluidic bacterial separation from blood. Nano Lett. 14(1), 1–5 (2014). https://doi.org/10.1021/nl3047305
X. Hou, X. Zhang, W. Zhao, C. Zeng, B. Deng et al., Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol. 15(1), 41–46 (2020). https://doi.org/10.1038/s41565-019-0600-1
J. Zhou, G. Yu, F. Huang, Supramolecular chemotherapy based on host-guest molecular recognition: a novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 46(22), 7021–7053 (2017). https://doi.org/10.1039/c6cs00898d
I.K. Herrmann, M. Urner, S. Graf, C.M. Schumacher, B. Roth-Z’graggen et al., Endotoxin removal by magnetic separation-based blood purification. Adv. Healthc. Mater. 2(6), 829–835 (2013). https://doi.org/10.1002/adhm.201200358
T. Liu, B. Xiao, F. Xiang, J. Tan, Z. Chen et al., Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 11(1), 2788 (2020). https://doi.org/10.1038/s41467-020-16544-7
M. Soh, D.W. Kang, H.G. Jeong, D. Kim, D.Y. Kim et al., Ceria-zirconia nanoparticles as an enhanced multi-antioxidant for sepsis treatment. Angew. Chem. Int. Ed. 56(38), 11399–11403 (2017). https://doi.org/10.1002/anie.201704904
V. Selvaraj, N.D. Manne, R. Arvapalli, K.M. Rice, G. Nandyala et al., Effect of cerium oxide nanoparticles on sepsis induced mortality and NF-kappaB signaling in cultured macrophages. Nanomedicine 10(8), 1275–1288 (2015). https://doi.org/10.2217/nnm.14.205
S. Asano, R. Arvapalli, N.D. Manne, M. Maheshwari, B. Ma et al., Cerium oxide nanoparticle treatment ameliorates peritonitis-induced diaphragm dysfunction. Int. J. Nanomed. 10(1), 6215–6225 (2015). https://doi.org/10.2147/IJN.S89783
S.K. Rajendrakumar, V. Revuri, M. Samidurai, A. Mohapatra, J.H. Lee et al., Peroxidase-mimicking nanoassembly mitigates lipopolysaccharide-induced endotoxemia and cognitive damage in the brain by impeding inflammatory signaling in macrophages. Nano Lett. 18(10), 6417–6426 (2018). https://doi.org/10.1021/acs.nanolett.8b02785
S. Thamphiwatana, P. Angsantikul, T. Escajadillo, Q. Zhang, J. Olson et al., Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc. Natl. Acad. Sci. USA 114(43), 11488–11493 (2017). https://doi.org/10.1073/pnas.1714267114
R. Molinaro, A. Pasto, C. Corbo, F. Taraballi, F. Giordano et al., Macrophage-derived nanovesicles exert intrinsic anti-inflammatory properties and prolong survival in sepsis through a direct interaction with macrophages. Nanoscale 11(28), 13576–13586 (2019). https://doi.org/10.1039/c9nr04253a
S. Shen, F. Han, A. Yuan, L. Wu, J. Cao et al., Engineered nanoparticles disguised as macrophages for trapping lipopolysaccharide and preventing endotoxemia. Biomaterials 189(1), 60–68 (2019). https://doi.org/10.1016/j.biomaterials.2018.10.029
V. Chhabria, S. Beeton, Development of nanosponges from erythrocyte ghosts for removal of streptolysin-o from mammalian blood. Nanomedicine 11(21), 2797–2807 (2016). https://doi.org/10.2217/nnm-2016-0180
Y. Chen, Y. Zhang, M. Chen, J. Zhuang, R.H. Fang et al., Biomimetic nanosponges suppress in vivo lethality induced by the whole secreted proteins of pathogenic bacteria. Small 15(6), e1804994 (2019). https://doi.org/10.1002/smll.201804994
G. Kibria, E.K. Ramos, Y. Wan, D.R. Gius, H. Liu, Exosomes as a drug delivery system in cancer therapy: potential and challenges. Mol. Pharmaceut. 15(9), 3625–3633 (2018). https://doi.org/10.1021/acs.molpharmaceut.8b00277
N. Terrasini, V. Lionetti, Exosomes in critical illness. Crit. Care Med. 45(6), 1054–1060 (2017). https://doi.org/10.1097/CCM.0000000000002328
J. Gao, S. Wang, Z. Wang, High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVS) for anti-inflammation therapy. Biomaterials 135, 62–73 (2017). https://doi.org/10.1016/j.biomaterials.2017.05.003
K.S. Park, K. Svennerholm, G.V. Shelke, E. Bandeira, C. Lasser et al., Mesenchymal stromal cell-derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via il-10. Stem Cell Res. Ther. 10(1), 231 (2019). https://doi.org/10.1186/s13287-019-1352-4
W. Lee, J. Seo, S. Kwak, E.J. Park, D.H. Na et al., A double-chambered protein nanocage loaded with thrombin receptor agonist peptide (TRAP) and gamma-carboxyglutamic acid of protein c (PC-Gla) for sepsis treatment. Adv. Mater. 27(42), 6637–6643 (2015). https://doi.org/10.1002/adma.201503093
C.Y. Zhang, X. Dong, J. Gao, W. Lin, Z. Liu et al., Nanoparticle-induced neutrophil apoptosis increasessurvival in sepsis and alleviates neurologicaldamage in stroke. Sci. Adv. 5, 7964 (2019). https://doi.org/10.1126/sciadv.aax7964
Y. Xin, M. Huang, W.W. Guo, Q. Huang, L.Z. Zhang et al., Nano-based delivery of RNAI in cancer therapy. Mol. Cancer 16(1), 134 (2017). https://doi.org/10.1186/s12943-017-0683-y
H. He, N. Zheng, Z. Song, K.H. Kim, C. Yao et al., Suppression of hepatic inflammation via systemic sirna delivery by membrane-disruptive and endosomolytic helical polypeptide hybrid nanoparticles. ACS Nano 10(2), 1859–1870 (2016). https://doi.org/10.1021/acsnano.5b05470
R. Penalva, C.J. Gonzalez-Navarro, C. Gamazo, I. Esparza, J.M. Irache, Zein nanoparticles for oral delivery of quercetin: pharmacokinetic studies and preventive anti-inflammatory effects in a mouse model of endotoxemia. Nanomed. Nanotechnol. 13(1), 103–110 (2017). https://doi.org/10.1016/j.nano.2016.08.033
S. Mura, J. Nicolas, P. Couvreur, Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12(11), 991–1003 (2013). https://doi.org/10.1038/nmat3776
L.M. Casey, S. Kakade, J.T. Decker, J.A. Rose, K. Deans et al., Cargo-less nanoparticles program innate immune cell responses to toll-like receptor activation. Biomaterials 218, 119333 (2019). https://doi.org/10.1016/j.biomaterials.2019.119333
S. Spence, M.K. Greene, F. Fay, E. Hams, S.P. Saunders et al., Targeting siglecs with a sialic acid–decoratednanoparticle abrogates inflammation. Sci. Transl. Med. 7(303), 303ra140 (2015). https://doi.org/10.1126/scitranslmed.aab3459
G. Li-Volti, T. Musumeci, R. Pignatello, P. Murabito, I. Barbagallo et al., Antioxidant potential of different melatonin-loaded nanomedicines in an experimental model of sepsis. Exp. Biol. Med. 237(6), 670–677 (2012). https://doi.org/10.1258/ebm.2012.011425
S. Zhang, J. Ermann, M.D. Succi, A. Zhou, M.J. Hamilton et al., An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci. Transl. Med. 7(300), 300128 (2015). https://doi.org/10.1126/scitranslmed.aaa5657
N. Joshi, J. Yan, S. Levy, S. Bhagchandani, K.V. Slaughter et al., Towards an arthritis flare-responsive drug delivery system. Nat. Commun. 9(1), 1275 (2018). https://doi.org/10.1038/s41467-018-03691-1
S.B. Lim, I. Rubinstein, R.T. Sadikot, J.E. Artwohl, H. Onyuksel, A novel peptide nanomedicine against acute lung injury: Glp-1 in phospholipid micelles. Pharm. Res. 28(3), 662–672 (2011). https://doi.org/10.1007/s11095-010-0322-4
M.C. Ferrer, V.V. Shuvaev, B.J. Zern, R.J. Composto, V.R. Muzykantov et al., ICAM-1 targeted nanogels loaded with dexamethasone alleviate pulmonary inflammation. PLoS ONE 9(7), e102329 (2014). https://doi.org/10.1371/journal.pone.0102329
E.B. Okeke, C. Louttit, C. Fry, A.H. Najafabadi, K. Han et al., Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials 238(1), 119836 (2020). https://doi.org/10.1016/j.biomaterials.2020.119836
C.Y. Zhang, J. Gao, Z. Wang, Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv. Mater. 30(43), e1803618 (2018). https://doi.org/10.1002/adma.201803618
Y. Yang, Y. Ding, B. Fan, Y. Wang, Z. Mao et al., Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J. Control. Release 321, 463–474 (2020). https://doi.org/10.1016/j.jconrel.2020.02.030
W. Lee, E.J. Park, G. Min, J. Choi, D.H. Na et al., Dual functioned pegylated phospholipid micelles containing cationic antimicrobial decapeptide for treating sepsis. Theranostics 7(15), 3759–3767 (2017). https://doi.org/10.7150/thno.20734
L. Tang, Z. Wang, Q. Mu, Z. Yu, O. Jacobson et al., Targeting neutrophils for enhanced cancer theranostics. Adv. Mater. 32(33), e2002739 (2020). https://doi.org/10.1002/adma.202002739
S. Wang, J. Lv, S. Meng, J. Tang, L. Nie, Recent advances in nanotheranostics for treat-to-target of rheumatoid arthritis. Adv. Healthc. Mater. 9(6), e1901541 (2020). https://doi.org/10.1002/adhm.201901541
B. Ma, H. Xu, W. Zhuang, Y. Wang, G. Li et al., Reactive oxygen species responsive theranostic nanoplatform for two-photon aggregation-induced emission imaging and therapy of acute and chronic inflammation. ACS Nano 14(5), 5862–5873 (2020). https://doi.org/10.1021/acsnano.0c01012
E.V. Fuior, C.A. Mocanu, M. Deleanu, G. Voicu, M. Anghelache et al., Evaluation of VCAM-1 targeted naringenin/indocyanine green-loaded lipid nanoemulsions as theranostic nanoplatforms in inflammation. Pharmaceutics 12(11), 1066 (2020). https://doi.org/10.3390/pharmaceutics12111066
D. Mao, F. Hu, S. Ji, W. Wu, D. Ding et al., Metal-organic-framework-assisted in vivo bacterial metabolic labeling and precise antibacterial therapy. Adv. Mater. 30(18), e1706831 (2018). https://doi.org/10.1002/adma.201706831
C. Shi, X. Wang, L. Wang, Q. Meng, D. Guo et al., A nanotrap improves survival in severe sepsis by attenuating hyperinflammation. Nat. Commun. 11(1), 3384 (2020). https://doi.org/10.1038/s41467-020-17153-0
M.I. Setyawati, C.Y. Tay, D. Docter, R.H. Stauber, D.T. Leong, Understanding and exploiting nanoparticles’ intimacy with the blood vessel and blood. Chem. Soc. Rev. 44(22), 8174–8199 (2015). https://doi.org/10.1039/c5cs00499c
M. Wang, J. Li, S. Dong, X. Cai, A. Simaiti et al., Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction. Part. Fibre Toxicol. 17(1), 23 (2020). https://doi.org/10.1186/s12989-020-00353-3