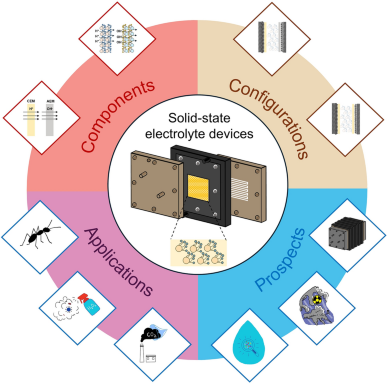
Electrochemical Solid-State Electrolyte Reactors: Configurations, Applications, and Future Prospects
Corresponding Author: Xiao Zhang
Nano-Micro Letters,
Vol. 17 (2025), Article Number: 306
Abstract
The advancement of clean electricity is positioning electrochemical reactors at the forefront of future electrosynthesis technologies. Solid-state electrolyte (SSE) reactors emerge for their distinctive configurations and ability to produce high-purity fuels and chemicals efficiently without additional purification steps. This marks a substantial development in electrochemical synthesis. In this perspective, we critically examine cutting-edge innovations in SSE devices with particular emphasis on the architectural introduction of core cell components, novel electrochemical cell configurations, and assembly methodologies. The use of SSE reactors is presently undergoing a pivotal transition from fundamental laboratory investigations to large-scale engineering implementations, demonstrating remarkable progress in multiple domains: (1) sustainable synthesis of high-value organic acids (formic and acetic acids), (2) production of critical oxidizers hydrogen peroxide (H2O2) and liquid fuels (ethanol), (3) ammonia (NH3) production, (4) carbon capture technologies, (5) lithium recovery and recycling, and (6) tandem or coupling strategies for high-value-added products. Importantly, the transformative potential in environmental remediation, particularly for airborne pollutant sequestration and advanced wastewater purification, is addressed. Additionally, the innovative architectural blueprints for next-generation SSE stack are presented, aiming to establish a comprehensive framework to guide the transition from laboratory-scale innovation to industrial-scale deployment of SSE devices in the foreseeable future.
Highlights:
1 Solid-state electrolyte reactors are elucidated in terms of their distinctive electrochemical architecture to facilitate the efficient direct synthesis of fuels and chemicals without the need for traditional purification steps.
2 The core components, variable configurations, and distinct electrochemical reaction mechanisms of different chambers are systematically summarized.
3 Potential future application scenarios and advanced cell stack designs are pointed out.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- J.H. Krenz, Energy: conversion and utilization. NASA STI/Recon. Tech. Rep. A 77, 14957 (1976).
- F. Shi, Reactor and Process Design in Sustainable Energy Technology (Elsevier, New York, 2014).
- G. Prentice, Electrochemical engineering. In Encyclopedia of physical science and technology (Academic Press, Cambridge, 2003), pp. 143–159.
- D. Pletcher, F.C. Walsh, Industrial Electrochemistry (Springer, Berlin, 2012).
- K. Scott, Sustainable and Green Electrochemical Science and Technology (Wiley, New York, 2017).
- A. Ursua, L.M. Gandia, P. Sanchis, Hydrogen production from water electrolysis: current status and future trends. Proc. IEEE 100(2), 410–426 (2012). https://doi.org/10.1109/JPROC.2011.2156750
- L. Fan, C. Xia, F. Yang, J. Wang, H. Wang et al., Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products. Sci. Adv. 6(8), eaay3111 (2020). https://doi.org/10.1126/sciadv.aay3111
- C. Xia, Y. Xia, P. Zhu, L. Fan, H. Wang, Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 366(6462), 226–231 (2019). https://doi.org/10.1126/science.aay1844
- C. Xia, P. Zhu, Q. Jiang, Y. Pan, W. Liang et al., Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 4(9), 776–785 (2019). https://doi.org/10.1038/s41560-019-0451-x
- T.-U. Wi, R. University, Z.H. Levell, S. Hao et al., Selective and stable ethanol synthesis via electrochemical CO2 reduction in a solid electrolyte reactor. ACS Energy Lett. 10(2), 822–829 (2025). https://doi.org/10.1021/acsenergylett.4c03091
- F.-Y. Chen, A. Elgazzar, S. Pecaut, C. Qiu, Y. Feng et al., Electrochemical nitrate reduction to ammonia with cation shuttling in a solid electrolyte reactor. Nat. Catal. 7(9), 1032–1043 (2024). https://doi.org/10.1038/s41929-024-01200-w
- P. Zhu, Z.Y. Wu, A. Elgazzar, C. Dong, T.U. Wi et al., Continuous carbon capture in an electrochemical solid-electrolyte reactor. Nature 618(7967), 959–966 (2023). https://doi.org/10.1038/s41586-023-06060-1
- Y. Feng, Y. Park, S. Hao, Z. Fang, T. Terlier et al., Three-chamber electrochemical reactor for selective lithium extraction from brine. Proc. Natl. Acad. Sci. U.S.A. 121(47), e2410033121 (2024). https://doi.org/10.1073/pnas.2410033121
- Z. Fang, P. Zhu, X. Zhang, Y. Feng, H. Wang, Self-looped electrochemical recycling of lithium-ion battery cathode materials to manufacturing feedstocks. Nat. Chem. Eng. 2(2), 142–151 (2025). https://doi.org/10.1038/s44286-025-00186-x
- P. Zhu, C. Xia, C.-Y. Liu, K. Jiang, G. Gao et al., Direct and continuous generation of pure acetic acid solutions via electrocatalytic carbon monoxide reduction. Proc. Natl. Acad. Sci. U.S.A. 118(2), e2010868118 (2021). https://doi.org/10.1073/pnas.2010868118
- X. Yan, M. Zhang, Y. Chen, Y. Wu, R. Wu et al., Synergy of Cu/C3N4 interface and Cu nanops dual catalytic regions in electrolysis of CO to acetic acid. Angew. Chem. Int. Ed. 62(22), e202301507 (2023). https://doi.org/10.1002/anie.202301507
- T. Zheng, C. Liu, C. Guo, M. Zhang, X. Li et al., Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nat. Nanotechnol. 16(12), 1386–1393 (2021). https://doi.org/10.1038/s41565-021-00974-5
- T.-U. Wi, Y. Xie, Z.H. Levell, D. Feng, J.Y. Kim et al., Upgrading carbon monoxide to bioplastics via integrated electrochemical reduction and biosynthesis. Nat. Synth. 3(11), 1392–1403 (2024). https://doi.org/10.1038/s44160-024-00621-6
- L. Fan, Y. Zhao, L. Chen, J. Chen, J. Chen et al., Selective production of ethylene glycol at high rate via cascade catalysis. Nat. Catal. 6(7), 585–595 (2023). https://doi.org/10.1038/s41929-023-00977-6
- S.-K. Zhang, Y. Feng, A. Elgazzar, Y. Xia, C. Qiu et al., Interfacial electrochemical-chemical reaction coupling for efficient olefin oxidation to glycols. Joule 7(8), 1887–1901 (2023). https://doi.org/10.1016/j.joule.2023.06.022
- T. Zheng, M. Zhang, L. Wu, S. Guo, X. Liu et al., Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering. Nat. Catal. 5(5), 388–396 (2022). https://doi.org/10.1038/s41929-022-00775-6
- W.D. Wetzels, Johann Wilhelm Ritter: Romantic physics in Germany. Romanticism and the Sciences 199–212 (1990).
- M. Paidar, V. Fateev, K. Bouzek, Membrane electrolysis: history, current status and perspective. Electrochim. Acta 209, 737–756 (2016). https://doi.org/10.1016/j.electacta.2016.05.209
- C. Oloman, Trickle bed electrochemical reactors. J. Electrochem. Soc. 126(11), 1885–1892 (1979). https://doi.org/10.1149/1.2128820
- K. Otsuka, I. Yamanaka, Electrochemical cells as reactors for selective oxygenation of hydrocarbons at low temperature. Catal. Today 41(4), 311–325 (1998). https://doi.org/10.1016/S0920-5861(98)00022-4
- E. Brillas, F. Alcaide, P.-L. Cabot, A small-scale flow alkaline fuel cell for on-site production of hydrogen peroxide. Electrochim. Acta 48(4), 331–340 (2002). https://doi.org/10.1016/S0013-4686(02)00665-5
- T. Murayama, I. Yamanaka, Electrosynthesis of neutral H2O2 solution from O2 and water at a mixed carbon cathode using an exposed solid-polymer-electrolyte electrolysis cell. J. Phys. Chem. C 115(13), 5792–5799 (2011). https://doi.org/10.1021/jp1109702
- Z. Chen, S. Chen, S. Siahrostami, P. Chakthranont, C. Hahn et al., Development of a reactor with carbon catalysts for modular-scale, low-cost electrochemical generation of H2O2. React. Chem. Eng. 2(2), 239–245 (2017). https://doi.org/10.1039/C6RE00195E
- J. Lee, Y. Tak, Electrocatalytic activity of Cu electrode in electroreduction of CO2. Electrochim. Acta 46(19), 3015–3022 (2001). https://doi.org/10.1016/S0013-4686(01)00527-8
- D.T. Whipple, E.C. Finke, P.J.A. Kenis, Microfluidic reactor for the electrochemical reduction of carbon dioxide: the effect of pH. Electrochem. Solid-State Lett. 13(9), B109 (2010). https://doi.org/10.1149/1.3456590
- C.T. Dinh, T. Burdyny, M.G. Kibria, A. Seifitokaldani, C.M. Gabardo et al., CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360(6390), 783–787 (2018). https://doi.org/10.1126/science.aas9100
- W. Lee, Y.E. Kim, M.H. Youn, S.K. Jeong, K.T. Park, Catholyte-free electrocatalytic CO2 reduction to formate. Angew. Chem. Int. Ed. 57(23), 6883–6887 (2018). https://doi.org/10.1002/anie.201803501
- J. Lee, J. Lim, C.-W. Roh, H.S. Whang, H. Lee, Electrochemical CO2 reduction using alkaline membrane electrode assembly on various metal electrodes. J. CO2 Util. 31, 244–250 (2019). https://doi.org/10.1016/j.jcou.2019.03.022
- C.-T. Dinh, F.P. García de Arquer, D. Sinton, E.H. Sargent, High rate, selective, and stable electroreduction of CO2 to CO in basic and neutral media. ACS Energy Lett. 3(11), 2835–2840 (2018). https://doi.org/10.1021/acsenergylett.8b01734
- R. Kortlever, I. Peters, S. Koper, M.T.M. Koper, Electrochemical CO2 reduction to formic acid at low overpotential and with high faradaic efficiency on carbon-supported bimetallic Pd–Pt nanops. ACS Catal. 5(7), 3916–3923 (2015). https://doi.org/10.1021/acscatal.5b00602
- W. Luc, X. Fu, J. Shi, J.-J. Lv, M. Jouny et al., Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2(5), 423–430 (2019). https://doi.org/10.1038/s41929-019-0269-8
- W. Liu, P. Zhai, A. Li, B. Wei, K. Si et al., Electrochemical CO2 reduction to ethylene by ultrathin CuO nanoplate arrays. Nat. Commun. 13(1), 1877 (2022). https://doi.org/10.1038/s41467-022-29428-9
- C.M. Gabardo, C.P. O’Brien, J.P. Edwards, C. McCallum, Y. Xu et al., Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly. Joule 3(11), 2777–2791 (2019). https://doi.org/10.1016/j.joule.2019.07.021
- B. Chang, H. Pang, F. Raziq, S. Wang, K.-W. Huang et al., Electrochemical reduction of carbon dioxide to multicarbon (C2+) products: challenges and perspectives. Energy Environ. Sci. 16(11), 4714–4758 (2023). https://doi.org/10.1039/d3ee00964e
- W. Li, Y. Zhai, Q. Xia, X. Zhang, An emerging solid-state electrolyte reactor to drive the future of electrochemical synthesis. Adv. Energy Mater. 14(48), 2403841 (2024). https://doi.org/10.1002/aenm.202403841
- C. Liu, Y. Ji, T. Zheng, C. Xia, Solid-state-electrolyte reactor: new opportunity for electrifying manufacture. JACS Au 5(2), 521–535 (2025). https://doi.org/10.1021/jacsau.4c01183
- P. Zhu, H. Wang, High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat. Catal. 4(11), 943–951 (2021). https://doi.org/10.1038/s41929-021-00694-y
- L. Fan, C. Xia, P. Zhu, Y. Lu, H. Wang, Electrochemical CO2 reduction to high-concentration pure formic acid solutions in an all-solid-state reactor. Nat. Commun. 11(1), 3633 (2020). https://doi.org/10.1038/s41467-020-17403-1
- X. Zhang, X. Zhao, P. Zhu, Z. Adler, Z.-Y. Wu et al., Electrochemical oxygen reduction to hydrogen peroxide at practical rates in strong acidic media. Nat. Commun. 13(1), 2880 (2022). https://doi.org/10.1038/s41467-022-30337-0
- X. Zhang, Z. Fang, P. Zhu, Y. Xia, H. Wang, Electrochemical regeneration of high-purity CO2 from (bi)carbonates in a porous solid electrolyte reactor for efficient carbon capture. Nat. Energy 10(1), 55–65 (2024). https://doi.org/10.1038/s41560-024-01654-z
- A. Varon, W. Rieman III., Kinetics of ion exchange in a chelating resin. J. Phys. Chem. 68(9), 2716–2718 (1964). https://doi.org/10.1021/j100791a503
- F. Sagane, T. Abe, Y. Iriyama, Z. Ogumi, Li+ and Na+ transfer through interfaces between inorganic solid electrolytes and polymer or liquid electrolytes. J. Power. Sources 146(1–2), 749–752 (2005). https://doi.org/10.1016/j.jpowsour.2005.03.075
- J.R. Millar, D.G. Smith, W.E. Marr, T.R.E. Kressman, Solvent-modified polymer networks. Part I. The preparation and characterisation of expanded-network and macroporous styrene–divinylbenzene copolymers and their sulphonates. J. Chem. Soc. (1963). https://doi.org/10.1039/jr9630000218
- K.A. Kun, R. Kunin, Macroreticular resins. III. Formation of macroreticular styrene–divinylbenzene copolymers. J. Polym. Sci. Part A 1 Polym. Chem. 6(10), 2689–2701 (1968). https://doi.org/10.1002/pol.1968.150061001
- J.-J. Woo, S.-J. Seo, S.-H. Yun, R.-Q. Fu, T.-H. Yang et al., Enhanced stability and proton conductivity of sulfonated polystyrene/PVC composite membranes through proper copolymerization of styrene with α-methylstyrene and acrylonitrile. J. Membr. Sci. 363(1–2), 80–86 (2010). https://doi.org/10.1016/j.memsci.2010.07.009
- S.H. Lee, J.C. Rasaiah, Proton transfer and the mobilities of the H+ and OH− ions from studies of a dissociating model for water. J. Chem. Phys. 135(12), 124505 (2011). https://doi.org/10.1063/1.3632990
- R. Kunin, R.J. Myers, The anion exchange equilibria in an anion exchange resin. J. Am. Chem. Soc. 69(11), 2874–2878 (1947). https://doi.org/10.1021/ja01203a070
- R.M. Wheaton, W.C. Bauman, Properties of strongly basic anion exchange resins. Ind. Eng. Chem. 43(5), 1088–1093 (1951). https://doi.org/10.1021/ie50497a027
- Y. Hu, J. Foster, T.H. Boyer, Selectivity of bicarbonate-form anion exchange for drinking water contaminants: Influence of resin properties. Sep. Purif. Technol. 163, 128–139 (2016). https://doi.org/10.1016/j.seppur.2016.02.030
- M.V. Rouilly, E.R. Kötz, O. Haas, G.G. Scherer, A. Chapiró, Proton exchange membranes prepared by simultaneous radiation grafting of styrene onto Teflon-FEP films. Synthesis and characterization. J. Membr. Sci. 81(1–2), 89–95 (1993). https://doi.org/10.1016/0376-7388(93)85033-S
- M.M. Nasef, H. Saidi, H.M. Nor, O.M. Foo, Proton exchange membranes prepared by simultaneous radiation grafting of styrene onto poly(tetrafluoroethylene-co-hexafluoropropylene) films. II. Properties of sulfonated membranes. J. Appl. Polym. Sci. 78(14), 2443–2453 (2000). https://doi.org/10.1002/1097-4628(20001227)
- R. Paterson, C.R. Gardner, Comparison of the transport properties of normal and expanded forms of a cation-exchange membrane by use of an irreversible thermodynamic approach. Part I. Membranes in the sodium form in 0·1M-sodium chloride. J. Chem. Soc. A (1971). https://doi.org/10.1039/j19710002254
- M. Ersöz, Diffusion and selective transport of alkali cations on cation-exchange membrane. Sep. Sci. Technol. 30(18), 3523–3533 (1995). https://doi.org/10.1080/01496399508015133
- I. Gatto, A. Caprì, C. Lo Vecchio, S. Zignani, A. Patti et al., Optimal operating conditions evaluation of an anion-exchange-membrane electrolyzer based on FUMASEP® FAA3–50 membrane. Int. J. Hydrog. Energy 48(32), 11914–11921 (2023). https://doi.org/10.1016/j.ijhydene.2022.04.176
- B. Motealleh, Z. Liu, R.I. Masel, J.P. Sculley, Z. Richard Ni et al., Next-generation anion exchange membrane water electrolyzers operating for commercially relevant lifetimes. Int. J. Hydrog. Energy 46(5), 3379–3386 (2021). https://doi.org/10.1016/j.ijhydene.2020.10.244
- J. Wang, Y. Zhao, B.P. Setzler, S. Rojas-Carbonell, C. Ben Yehuda et al., Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat. Energy 4(5), 392–398 (2019). https://doi.org/10.1038/s41560-019-0372-8
- S. Garg, C.A. Giron Rodriguez, T.E. Rufford, J.R. Varcoe, B. Seger, How membrane characteristics influence the performance of CO2 and CO electrolysis. Energy Environ. Sci. 15(11), 4440–4469 (2022). https://doi.org/10.1039/D2EE01818G
- X. Luo, D.I. Kushner, A. Kusoglu, Anion exchange membranes: The effect of reinforcement in water and electrolyte. J. Membr. Sci. 685, 121945 (2023). https://doi.org/10.1016/j.memsci.2023.121945
- K. Peramaiah, M. Yi, I. Dutta, S. Chatterjee, H. Zhang et al., Catalyst design and engineering for CO2-to-formic acid electrosynthesis for a low-carbon economy. Adv. Mater. 36(51), 2470410 (2024). https://doi.org/10.1002/adma.202470410
- X. Wu, H. Zhang, S. Zuo, J. Dong, Y. Li et al., Engineering the coordination sphere of isolated active sites to explore the intrinsic activity in single-atom catalysts. Nano-Micro Lett. 13(1), 136 (2021). https://doi.org/10.1007/s40820-021-00668-6
- J. Yu, Z. Song, Q. Qi, X. Hui, Y. Ma et al., Sabatier principle inspired bifunctional alloy interface for stable and high-depth discharging zinc metal anodes. Angew. Chem. Int. Ed. 64(15), e202423236 (2025). https://doi.org/10.1002/anie.202423236
- Y. Liu, C. Wang, Y. Lei, F. Liu, B. Tian et al., Investigation of high-performance IrO2 electrocatalysts prepared by Adams method. Int. J. Hydrog. Energy 43(42), 19460–19467 (2018). https://doi.org/10.1016/j.ijhydene.2018.08.196
- L. She, G. Zhao, T. Ma, J. Chen, W. Sun et al., On the durability of iridium-based electrocatalysts toward the oxygen evolution reaction under acid environment. Adv. Funct. Mater. 32(5), 2108465 (2022). https://doi.org/10.1002/adfm.202108465
- T.-F. Ko, P.-W. Chen, K.-M. Li, H.-T. Young, C.-T. Chang et al., High-performance complementary electrochromic device based on iridium oxide as a counter electrode. Materials 14(7), 1591 (2021). https://doi.org/10.3390/ma14071591
- M.V. Williams, E. Begg, L. Bonville, H.R. Kunz, J.M. Fenton, Characterization of gas diffusion layers for PEMFC. J. Electrochem. Soc. 151(8), A1173 (2004). https://doi.org/10.1149/1.1764779
- N. Zamel, X. Li, Effective transport properties for polymer electrolyte membrane fuel cells–With a focus on the gas diffusion layer. Prog. Energy Combust. Sci. 39(1), 111–146 (2013). https://doi.org/10.1016/j.pecs.2012.07.002
- R.L. Borup, N.E. Vanderborgh, Design and testing criteria for bipolar plate materials for pem fuel cell applications. MRS Online Proc. Libr. 393(1), 151–155 (1995). https://doi.org/10.1557/PROC-393-151
- S.-H. Wang, J. Peng, W.-B. Lui, Surface modification and development of titanium bipolar plates for PEM fuel cells. J. Power. Sources 160(1), 485–489 (2006). https://doi.org/10.1016/j.jpowsour.2006.01.020
- Y. Leng, P. Ming, D. Yang, C. Zhang, Stainless steel bipolar plates for proton exchange membrane fuel cells: materials, flow channel design and forming processes. J. Power. Sources 451, 227783 (2020). https://doi.org/10.1016/j.jpowsour.2020.227783
- J. Wang, Theory and practice of flow field designs for fuel cell scaling-up: a critical review. Appl. Energy 157, 640–663 (2015). https://doi.org/10.1016/j.apenergy.2015.01.032
- J. Shen, Z. Tu, S.H. Chan, Enhancement of mass transfer in a proton exchange membrane fuel cell with blockage in the flow channel. Appl. Therm. Eng. 149, 1408–1418 (2019). https://doi.org/10.1016/j.applthermaleng.2018.12.138
- W. Pan, P. Wang, X. Chen, F. Wang, G. Dai, Combined effects of flow channel configuration and operating conditions on PEM fuel cell performance. Energy Convers. Manag. 220, 113046 (2020). https://doi.org/10.1016/j.enconman.2020.113046
- A. Elgazzar, H. Wang, Beyond molecular transformations in electrochemical porous solid electrolyte reactors. Nat. Chem. Eng. 2(1), 3–7 (2025). https://doi.org/10.1038/s44286-024-00160-z
- G.V. Samsonov, V.A. Pasechnik, Ion exchange and the swelling of ion-exchange resins. Russ. Chem. Rev. 38(7), 547–565 (1969). https://doi.org/10.1070/rc1969v038n07abeh001761
- J. Karo, A. Aabloo, J.O. Thomas, D. Brandell, Molecular dynamics modeling of proton transport in nafion and hyflon nanostructures. J. Phys. Chem. B 114(18), 6056–6064 (2010). https://doi.org/10.1021/jp903288y
- Y. Xia, X. Zhao, C. Xia, Z.Y. Wu, P. Zhu et al., Highly active and selective oxygen reduction to H2O2 on boron-doped carbon for high production rates. Nat. Commun. 12(1), 4225 (2021). https://doi.org/10.1038/s41467-021-24329-9
- J.Y. Kim, P. Zhu, F.-Y. Chen, Z.-Y. Wu, D.A. Cullen et al., Recovering carbon losses in CO2 electrolysis using a solid electrolyte reactor. Nat. Catal. 5(4), 288–299 (2022). https://doi.org/10.1038/s41929-022-00763-w
- H.D. Willauer, F. DiMascio, D.R. Hardy, M.K. Lewis, F.W. Williams, Development of an electrochemical acidification cell for the recovery of CO2 and H2 from seawater. Ind. Eng. Chem. Res. 50(17), 9876–9882 (2011). https://doi.org/10.1021/ie2008136
- B. Sabri Rawah, M. Albloushi, W. Li, Electro-synthesis of pure aqueous H2O2 on nitrogen-doped carbon in a solid electrolyte flow cell without using anion exchange membrane. Chem. Eng. J. 466, 143282 (2023). https://doi.org/10.1016/j.cej.2023.143282
- H. Yang, J.J. Kaczur, S.D. Sajjad, R.I. Masel, Performance and long-term stability of CO2 conversion to formic acid using a three-compartment electrolyzer design. J. CO2 Util. 42, 101349 (2020). https://doi.org/10.1016/j.jcou.2020.101349
- H. Yang, J.J. Kaczur, S.D. Sajjad, R.I. Masel, Electrochemical conversion of CO2 to formic acid utilizing Sustainion™ membranes. J. CO2 Util. 20, 208–217 (2017). https://doi.org/10.1016/j.jcou.2017.04.011
- J. Zhu, J. Li, R. Lu, R. Yu, S. Zhao et al., Surface passivation for highly active, selective, stable, and scalable CO2 electroreduction. Nat. Commun. 14(1), 4670 (2023). https://doi.org/10.1038/s41467-023-40342-6
- Y. Xia, R. University, P. Zhu, R. University, Y. Yang et al., Electrochemical manufacturing of hydrogen peroxide with high concentration and durability. ACS Catal. 15(6), 4560–4569 (2025). https://doi.org/10.1021/acscatal.4c07033
- D. Zhou, L. Wang, F. Zhang, J. Wu, H. Wang et al., Feasible degradation of polyethylene terephthalate fiber-based microplastics in alkaline media with Bi2O3@N-TiO2 Z-scheme photocatalytic system. Adv. Sustain. Syst. 6(5), 2100516 (2022). https://doi.org/10.1002/adsu.202100516
- M. Dilara Hatinoglu, F. Dilek Sanin, Fate and effects of polyethylene terephthalate (PET) microplastics during anaerobic digestion of alkaline-thermal pretreated sludge. Waste Manag. 153, 376–385 (2022). https://doi.org/10.1016/j.wasman.2022.09.016
- S. Miao, B.H. Shanks, Mechanism of acetic acid esterification over sulfonic acid-functionalized mesoporous silica. J. Catal. 279(1), 136–143 (2011). https://doi.org/10.1016/j.jcat.2011.01.008
- R.A. Ahmed, S. Rashid, K. Huddersman, Esterification of stearic acid using novel protonated and crosslinked amidoximated polyacrylonitrile ion exchange fibres. J. Ind. Eng. Chem. 119, 550–573 (2023). https://doi.org/10.1016/j.jiec.2022.12.001
- F. Barbir, Fuel Cell Stack Design Principles with Some Design Concepts of Micro-Mini Fuel Cells (Springer, Netherlands, 2008), pp.27–46. https://doi.org/10.1007/978-1-4020-8295-5_3
- S. Kakaç, A. Pramuanjaroenkij, L. Vasiliev, Mini-Micro Fuel Cells: Fundamentals and Applications (Springer, Berlin, 2008)
- J. Mergel, D.L. Fritz, M. Carmo, Stack technology for PEM electrolysis, in Hydrogen Science and Engineering: Materials, Processes, Systems and Technology (Wiley‐VCH Verlag GmbH & Co. KGaA, 2016), pp. 331–358. https://doi.org/10.1002/9783527674268.ch15
- E. Zhao, Y. Zhang, J. Zhan, G. Xia, G. Yu et al., Optimization and scaling-up of porous solid electrolyte electrochemical reactors for hydrogen peroxide electrosynthesis. Nat. Commun. 16(1), 3212 (2025). https://doi.org/10.1038/s41467-025-58385-2
- C.-H. Chen, S.-P. Jung, S.-C. Yen, Flow distribution in the manifold of PEM fuel cell stack. J. Power. Sources 173(1), 249–263 (2007). https://doi.org/10.1016/j.jpowsour.2007.05.007
References
J.H. Krenz, Energy: conversion and utilization. NASA STI/Recon. Tech. Rep. A 77, 14957 (1976).
F. Shi, Reactor and Process Design in Sustainable Energy Technology (Elsevier, New York, 2014).
G. Prentice, Electrochemical engineering. In Encyclopedia of physical science and technology (Academic Press, Cambridge, 2003), pp. 143–159.
D. Pletcher, F.C. Walsh, Industrial Electrochemistry (Springer, Berlin, 2012).
K. Scott, Sustainable and Green Electrochemical Science and Technology (Wiley, New York, 2017).
A. Ursua, L.M. Gandia, P. Sanchis, Hydrogen production from water electrolysis: current status and future trends. Proc. IEEE 100(2), 410–426 (2012). https://doi.org/10.1109/JPROC.2011.2156750
L. Fan, C. Xia, F. Yang, J. Wang, H. Wang et al., Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products. Sci. Adv. 6(8), eaay3111 (2020). https://doi.org/10.1126/sciadv.aay3111
C. Xia, Y. Xia, P. Zhu, L. Fan, H. Wang, Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 366(6462), 226–231 (2019). https://doi.org/10.1126/science.aay1844
C. Xia, P. Zhu, Q. Jiang, Y. Pan, W. Liang et al., Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 4(9), 776–785 (2019). https://doi.org/10.1038/s41560-019-0451-x
T.-U. Wi, R. University, Z.H. Levell, S. Hao et al., Selective and stable ethanol synthesis via electrochemical CO2 reduction in a solid electrolyte reactor. ACS Energy Lett. 10(2), 822–829 (2025). https://doi.org/10.1021/acsenergylett.4c03091
F.-Y. Chen, A. Elgazzar, S. Pecaut, C. Qiu, Y. Feng et al., Electrochemical nitrate reduction to ammonia with cation shuttling in a solid electrolyte reactor. Nat. Catal. 7(9), 1032–1043 (2024). https://doi.org/10.1038/s41929-024-01200-w
P. Zhu, Z.Y. Wu, A. Elgazzar, C. Dong, T.U. Wi et al., Continuous carbon capture in an electrochemical solid-electrolyte reactor. Nature 618(7967), 959–966 (2023). https://doi.org/10.1038/s41586-023-06060-1
Y. Feng, Y. Park, S. Hao, Z. Fang, T. Terlier et al., Three-chamber electrochemical reactor for selective lithium extraction from brine. Proc. Natl. Acad. Sci. U.S.A. 121(47), e2410033121 (2024). https://doi.org/10.1073/pnas.2410033121
Z. Fang, P. Zhu, X. Zhang, Y. Feng, H. Wang, Self-looped electrochemical recycling of lithium-ion battery cathode materials to manufacturing feedstocks. Nat. Chem. Eng. 2(2), 142–151 (2025). https://doi.org/10.1038/s44286-025-00186-x
P. Zhu, C. Xia, C.-Y. Liu, K. Jiang, G. Gao et al., Direct and continuous generation of pure acetic acid solutions via electrocatalytic carbon monoxide reduction. Proc. Natl. Acad. Sci. U.S.A. 118(2), e2010868118 (2021). https://doi.org/10.1073/pnas.2010868118
X. Yan, M. Zhang, Y. Chen, Y. Wu, R. Wu et al., Synergy of Cu/C3N4 interface and Cu nanops dual catalytic regions in electrolysis of CO to acetic acid. Angew. Chem. Int. Ed. 62(22), e202301507 (2023). https://doi.org/10.1002/anie.202301507
T. Zheng, C. Liu, C. Guo, M. Zhang, X. Li et al., Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nat. Nanotechnol. 16(12), 1386–1393 (2021). https://doi.org/10.1038/s41565-021-00974-5
T.-U. Wi, Y. Xie, Z.H. Levell, D. Feng, J.Y. Kim et al., Upgrading carbon monoxide to bioplastics via integrated electrochemical reduction and biosynthesis. Nat. Synth. 3(11), 1392–1403 (2024). https://doi.org/10.1038/s44160-024-00621-6
L. Fan, Y. Zhao, L. Chen, J. Chen, J. Chen et al., Selective production of ethylene glycol at high rate via cascade catalysis. Nat. Catal. 6(7), 585–595 (2023). https://doi.org/10.1038/s41929-023-00977-6
S.-K. Zhang, Y. Feng, A. Elgazzar, Y. Xia, C. Qiu et al., Interfacial electrochemical-chemical reaction coupling for efficient olefin oxidation to glycols. Joule 7(8), 1887–1901 (2023). https://doi.org/10.1016/j.joule.2023.06.022
T. Zheng, M. Zhang, L. Wu, S. Guo, X. Liu et al., Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering. Nat. Catal. 5(5), 388–396 (2022). https://doi.org/10.1038/s41929-022-00775-6
W.D. Wetzels, Johann Wilhelm Ritter: Romantic physics in Germany. Romanticism and the Sciences 199–212 (1990).
M. Paidar, V. Fateev, K. Bouzek, Membrane electrolysis: history, current status and perspective. Electrochim. Acta 209, 737–756 (2016). https://doi.org/10.1016/j.electacta.2016.05.209
C. Oloman, Trickle bed electrochemical reactors. J. Electrochem. Soc. 126(11), 1885–1892 (1979). https://doi.org/10.1149/1.2128820
K. Otsuka, I. Yamanaka, Electrochemical cells as reactors for selective oxygenation of hydrocarbons at low temperature. Catal. Today 41(4), 311–325 (1998). https://doi.org/10.1016/S0920-5861(98)00022-4
E. Brillas, F. Alcaide, P.-L. Cabot, A small-scale flow alkaline fuel cell for on-site production of hydrogen peroxide. Electrochim. Acta 48(4), 331–340 (2002). https://doi.org/10.1016/S0013-4686(02)00665-5
T. Murayama, I. Yamanaka, Electrosynthesis of neutral H2O2 solution from O2 and water at a mixed carbon cathode using an exposed solid-polymer-electrolyte electrolysis cell. J. Phys. Chem. C 115(13), 5792–5799 (2011). https://doi.org/10.1021/jp1109702
Z. Chen, S. Chen, S. Siahrostami, P. Chakthranont, C. Hahn et al., Development of a reactor with carbon catalysts for modular-scale, low-cost electrochemical generation of H2O2. React. Chem. Eng. 2(2), 239–245 (2017). https://doi.org/10.1039/C6RE00195E
J. Lee, Y. Tak, Electrocatalytic activity of Cu electrode in electroreduction of CO2. Electrochim. Acta 46(19), 3015–3022 (2001). https://doi.org/10.1016/S0013-4686(01)00527-8
D.T. Whipple, E.C. Finke, P.J.A. Kenis, Microfluidic reactor for the electrochemical reduction of carbon dioxide: the effect of pH. Electrochem. Solid-State Lett. 13(9), B109 (2010). https://doi.org/10.1149/1.3456590
C.T. Dinh, T. Burdyny, M.G. Kibria, A. Seifitokaldani, C.M. Gabardo et al., CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360(6390), 783–787 (2018). https://doi.org/10.1126/science.aas9100
W. Lee, Y.E. Kim, M.H. Youn, S.K. Jeong, K.T. Park, Catholyte-free electrocatalytic CO2 reduction to formate. Angew. Chem. Int. Ed. 57(23), 6883–6887 (2018). https://doi.org/10.1002/anie.201803501
J. Lee, J. Lim, C.-W. Roh, H.S. Whang, H. Lee, Electrochemical CO2 reduction using alkaline membrane electrode assembly on various metal electrodes. J. CO2 Util. 31, 244–250 (2019). https://doi.org/10.1016/j.jcou.2019.03.022
C.-T. Dinh, F.P. García de Arquer, D. Sinton, E.H. Sargent, High rate, selective, and stable electroreduction of CO2 to CO in basic and neutral media. ACS Energy Lett. 3(11), 2835–2840 (2018). https://doi.org/10.1021/acsenergylett.8b01734
R. Kortlever, I. Peters, S. Koper, M.T.M. Koper, Electrochemical CO2 reduction to formic acid at low overpotential and with high faradaic efficiency on carbon-supported bimetallic Pd–Pt nanops. ACS Catal. 5(7), 3916–3923 (2015). https://doi.org/10.1021/acscatal.5b00602
W. Luc, X. Fu, J. Shi, J.-J. Lv, M. Jouny et al., Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2(5), 423–430 (2019). https://doi.org/10.1038/s41929-019-0269-8
W. Liu, P. Zhai, A. Li, B. Wei, K. Si et al., Electrochemical CO2 reduction to ethylene by ultrathin CuO nanoplate arrays. Nat. Commun. 13(1), 1877 (2022). https://doi.org/10.1038/s41467-022-29428-9
C.M. Gabardo, C.P. O’Brien, J.P. Edwards, C. McCallum, Y. Xu et al., Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly. Joule 3(11), 2777–2791 (2019). https://doi.org/10.1016/j.joule.2019.07.021
B. Chang, H. Pang, F. Raziq, S. Wang, K.-W. Huang et al., Electrochemical reduction of carbon dioxide to multicarbon (C2+) products: challenges and perspectives. Energy Environ. Sci. 16(11), 4714–4758 (2023). https://doi.org/10.1039/d3ee00964e
W. Li, Y. Zhai, Q. Xia, X. Zhang, An emerging solid-state electrolyte reactor to drive the future of electrochemical synthesis. Adv. Energy Mater. 14(48), 2403841 (2024). https://doi.org/10.1002/aenm.202403841
C. Liu, Y. Ji, T. Zheng, C. Xia, Solid-state-electrolyte reactor: new opportunity for electrifying manufacture. JACS Au 5(2), 521–535 (2025). https://doi.org/10.1021/jacsau.4c01183
P. Zhu, H. Wang, High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat. Catal. 4(11), 943–951 (2021). https://doi.org/10.1038/s41929-021-00694-y
L. Fan, C. Xia, P. Zhu, Y. Lu, H. Wang, Electrochemical CO2 reduction to high-concentration pure formic acid solutions in an all-solid-state reactor. Nat. Commun. 11(1), 3633 (2020). https://doi.org/10.1038/s41467-020-17403-1
X. Zhang, X. Zhao, P. Zhu, Z. Adler, Z.-Y. Wu et al., Electrochemical oxygen reduction to hydrogen peroxide at practical rates in strong acidic media. Nat. Commun. 13(1), 2880 (2022). https://doi.org/10.1038/s41467-022-30337-0
X. Zhang, Z. Fang, P. Zhu, Y. Xia, H. Wang, Electrochemical regeneration of high-purity CO2 from (bi)carbonates in a porous solid electrolyte reactor for efficient carbon capture. Nat. Energy 10(1), 55–65 (2024). https://doi.org/10.1038/s41560-024-01654-z
A. Varon, W. Rieman III., Kinetics of ion exchange in a chelating resin. J. Phys. Chem. 68(9), 2716–2718 (1964). https://doi.org/10.1021/j100791a503
F. Sagane, T. Abe, Y. Iriyama, Z. Ogumi, Li+ and Na+ transfer through interfaces between inorganic solid electrolytes and polymer or liquid electrolytes. J. Power. Sources 146(1–2), 749–752 (2005). https://doi.org/10.1016/j.jpowsour.2005.03.075
J.R. Millar, D.G. Smith, W.E. Marr, T.R.E. Kressman, Solvent-modified polymer networks. Part I. The preparation and characterisation of expanded-network and macroporous styrene–divinylbenzene copolymers and their sulphonates. J. Chem. Soc. (1963). https://doi.org/10.1039/jr9630000218
K.A. Kun, R. Kunin, Macroreticular resins. III. Formation of macroreticular styrene–divinylbenzene copolymers. J. Polym. Sci. Part A 1 Polym. Chem. 6(10), 2689–2701 (1968). https://doi.org/10.1002/pol.1968.150061001
J.-J. Woo, S.-J. Seo, S.-H. Yun, R.-Q. Fu, T.-H. Yang et al., Enhanced stability and proton conductivity of sulfonated polystyrene/PVC composite membranes through proper copolymerization of styrene with α-methylstyrene and acrylonitrile. J. Membr. Sci. 363(1–2), 80–86 (2010). https://doi.org/10.1016/j.memsci.2010.07.009
S.H. Lee, J.C. Rasaiah, Proton transfer and the mobilities of the H+ and OH− ions from studies of a dissociating model for water. J. Chem. Phys. 135(12), 124505 (2011). https://doi.org/10.1063/1.3632990
R. Kunin, R.J. Myers, The anion exchange equilibria in an anion exchange resin. J. Am. Chem. Soc. 69(11), 2874–2878 (1947). https://doi.org/10.1021/ja01203a070
R.M. Wheaton, W.C. Bauman, Properties of strongly basic anion exchange resins. Ind. Eng. Chem. 43(5), 1088–1093 (1951). https://doi.org/10.1021/ie50497a027
Y. Hu, J. Foster, T.H. Boyer, Selectivity of bicarbonate-form anion exchange for drinking water contaminants: Influence of resin properties. Sep. Purif. Technol. 163, 128–139 (2016). https://doi.org/10.1016/j.seppur.2016.02.030
M.V. Rouilly, E.R. Kötz, O. Haas, G.G. Scherer, A. Chapiró, Proton exchange membranes prepared by simultaneous radiation grafting of styrene onto Teflon-FEP films. Synthesis and characterization. J. Membr. Sci. 81(1–2), 89–95 (1993). https://doi.org/10.1016/0376-7388(93)85033-S
M.M. Nasef, H. Saidi, H.M. Nor, O.M. Foo, Proton exchange membranes prepared by simultaneous radiation grafting of styrene onto poly(tetrafluoroethylene-co-hexafluoropropylene) films. II. Properties of sulfonated membranes. J. Appl. Polym. Sci. 78(14), 2443–2453 (2000). https://doi.org/10.1002/1097-4628(20001227)
R. Paterson, C.R. Gardner, Comparison of the transport properties of normal and expanded forms of a cation-exchange membrane by use of an irreversible thermodynamic approach. Part I. Membranes in the sodium form in 0·1M-sodium chloride. J. Chem. Soc. A (1971). https://doi.org/10.1039/j19710002254
M. Ersöz, Diffusion and selective transport of alkali cations on cation-exchange membrane. Sep. Sci. Technol. 30(18), 3523–3533 (1995). https://doi.org/10.1080/01496399508015133
I. Gatto, A. Caprì, C. Lo Vecchio, S. Zignani, A. Patti et al., Optimal operating conditions evaluation of an anion-exchange-membrane electrolyzer based on FUMASEP® FAA3–50 membrane. Int. J. Hydrog. Energy 48(32), 11914–11921 (2023). https://doi.org/10.1016/j.ijhydene.2022.04.176
B. Motealleh, Z. Liu, R.I. Masel, J.P. Sculley, Z. Richard Ni et al., Next-generation anion exchange membrane water electrolyzers operating for commercially relevant lifetimes. Int. J. Hydrog. Energy 46(5), 3379–3386 (2021). https://doi.org/10.1016/j.ijhydene.2020.10.244
J. Wang, Y. Zhao, B.P. Setzler, S. Rojas-Carbonell, C. Ben Yehuda et al., Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat. Energy 4(5), 392–398 (2019). https://doi.org/10.1038/s41560-019-0372-8
S. Garg, C.A. Giron Rodriguez, T.E. Rufford, J.R. Varcoe, B. Seger, How membrane characteristics influence the performance of CO2 and CO electrolysis. Energy Environ. Sci. 15(11), 4440–4469 (2022). https://doi.org/10.1039/D2EE01818G
X. Luo, D.I. Kushner, A. Kusoglu, Anion exchange membranes: The effect of reinforcement in water and electrolyte. J. Membr. Sci. 685, 121945 (2023). https://doi.org/10.1016/j.memsci.2023.121945
K. Peramaiah, M. Yi, I. Dutta, S. Chatterjee, H. Zhang et al., Catalyst design and engineering for CO2-to-formic acid electrosynthesis for a low-carbon economy. Adv. Mater. 36(51), 2470410 (2024). https://doi.org/10.1002/adma.202470410
X. Wu, H. Zhang, S. Zuo, J. Dong, Y. Li et al., Engineering the coordination sphere of isolated active sites to explore the intrinsic activity in single-atom catalysts. Nano-Micro Lett. 13(1), 136 (2021). https://doi.org/10.1007/s40820-021-00668-6
J. Yu, Z. Song, Q. Qi, X. Hui, Y. Ma et al., Sabatier principle inspired bifunctional alloy interface for stable and high-depth discharging zinc metal anodes. Angew. Chem. Int. Ed. 64(15), e202423236 (2025). https://doi.org/10.1002/anie.202423236
Y. Liu, C. Wang, Y. Lei, F. Liu, B. Tian et al., Investigation of high-performance IrO2 electrocatalysts prepared by Adams method. Int. J. Hydrog. Energy 43(42), 19460–19467 (2018). https://doi.org/10.1016/j.ijhydene.2018.08.196
L. She, G. Zhao, T. Ma, J. Chen, W. Sun et al., On the durability of iridium-based electrocatalysts toward the oxygen evolution reaction under acid environment. Adv. Funct. Mater. 32(5), 2108465 (2022). https://doi.org/10.1002/adfm.202108465
T.-F. Ko, P.-W. Chen, K.-M. Li, H.-T. Young, C.-T. Chang et al., High-performance complementary electrochromic device based on iridium oxide as a counter electrode. Materials 14(7), 1591 (2021). https://doi.org/10.3390/ma14071591
M.V. Williams, E. Begg, L. Bonville, H.R. Kunz, J.M. Fenton, Characterization of gas diffusion layers for PEMFC. J. Electrochem. Soc. 151(8), A1173 (2004). https://doi.org/10.1149/1.1764779
N. Zamel, X. Li, Effective transport properties for polymer electrolyte membrane fuel cells–With a focus on the gas diffusion layer. Prog. Energy Combust. Sci. 39(1), 111–146 (2013). https://doi.org/10.1016/j.pecs.2012.07.002
R.L. Borup, N.E. Vanderborgh, Design and testing criteria for bipolar plate materials for pem fuel cell applications. MRS Online Proc. Libr. 393(1), 151–155 (1995). https://doi.org/10.1557/PROC-393-151
S.-H. Wang, J. Peng, W.-B. Lui, Surface modification and development of titanium bipolar plates for PEM fuel cells. J. Power. Sources 160(1), 485–489 (2006). https://doi.org/10.1016/j.jpowsour.2006.01.020
Y. Leng, P. Ming, D. Yang, C. Zhang, Stainless steel bipolar plates for proton exchange membrane fuel cells: materials, flow channel design and forming processes. J. Power. Sources 451, 227783 (2020). https://doi.org/10.1016/j.jpowsour.2020.227783
J. Wang, Theory and practice of flow field designs for fuel cell scaling-up: a critical review. Appl. Energy 157, 640–663 (2015). https://doi.org/10.1016/j.apenergy.2015.01.032
J. Shen, Z. Tu, S.H. Chan, Enhancement of mass transfer in a proton exchange membrane fuel cell with blockage in the flow channel. Appl. Therm. Eng. 149, 1408–1418 (2019). https://doi.org/10.1016/j.applthermaleng.2018.12.138
W. Pan, P. Wang, X. Chen, F. Wang, G. Dai, Combined effects of flow channel configuration and operating conditions on PEM fuel cell performance. Energy Convers. Manag. 220, 113046 (2020). https://doi.org/10.1016/j.enconman.2020.113046
A. Elgazzar, H. Wang, Beyond molecular transformations in electrochemical porous solid electrolyte reactors. Nat. Chem. Eng. 2(1), 3–7 (2025). https://doi.org/10.1038/s44286-024-00160-z
G.V. Samsonov, V.A. Pasechnik, Ion exchange and the swelling of ion-exchange resins. Russ. Chem. Rev. 38(7), 547–565 (1969). https://doi.org/10.1070/rc1969v038n07abeh001761
J. Karo, A. Aabloo, J.O. Thomas, D. Brandell, Molecular dynamics modeling of proton transport in nafion and hyflon nanostructures. J. Phys. Chem. B 114(18), 6056–6064 (2010). https://doi.org/10.1021/jp903288y
Y. Xia, X. Zhao, C. Xia, Z.Y. Wu, P. Zhu et al., Highly active and selective oxygen reduction to H2O2 on boron-doped carbon for high production rates. Nat. Commun. 12(1), 4225 (2021). https://doi.org/10.1038/s41467-021-24329-9
J.Y. Kim, P. Zhu, F.-Y. Chen, Z.-Y. Wu, D.A. Cullen et al., Recovering carbon losses in CO2 electrolysis using a solid electrolyte reactor. Nat. Catal. 5(4), 288–299 (2022). https://doi.org/10.1038/s41929-022-00763-w
H.D. Willauer, F. DiMascio, D.R. Hardy, M.K. Lewis, F.W. Williams, Development of an electrochemical acidification cell for the recovery of CO2 and H2 from seawater. Ind. Eng. Chem. Res. 50(17), 9876–9882 (2011). https://doi.org/10.1021/ie2008136
B. Sabri Rawah, M. Albloushi, W. Li, Electro-synthesis of pure aqueous H2O2 on nitrogen-doped carbon in a solid electrolyte flow cell without using anion exchange membrane. Chem. Eng. J. 466, 143282 (2023). https://doi.org/10.1016/j.cej.2023.143282
H. Yang, J.J. Kaczur, S.D. Sajjad, R.I. Masel, Performance and long-term stability of CO2 conversion to formic acid using a three-compartment electrolyzer design. J. CO2 Util. 42, 101349 (2020). https://doi.org/10.1016/j.jcou.2020.101349
H. Yang, J.J. Kaczur, S.D. Sajjad, R.I. Masel, Electrochemical conversion of CO2 to formic acid utilizing Sustainion™ membranes. J. CO2 Util. 20, 208–217 (2017). https://doi.org/10.1016/j.jcou.2017.04.011
J. Zhu, J. Li, R. Lu, R. Yu, S. Zhao et al., Surface passivation for highly active, selective, stable, and scalable CO2 electroreduction. Nat. Commun. 14(1), 4670 (2023). https://doi.org/10.1038/s41467-023-40342-6
Y. Xia, R. University, P. Zhu, R. University, Y. Yang et al., Electrochemical manufacturing of hydrogen peroxide with high concentration and durability. ACS Catal. 15(6), 4560–4569 (2025). https://doi.org/10.1021/acscatal.4c07033
D. Zhou, L. Wang, F. Zhang, J. Wu, H. Wang et al., Feasible degradation of polyethylene terephthalate fiber-based microplastics in alkaline media with Bi2O3@N-TiO2 Z-scheme photocatalytic system. Adv. Sustain. Syst. 6(5), 2100516 (2022). https://doi.org/10.1002/adsu.202100516
M. Dilara Hatinoglu, F. Dilek Sanin, Fate and effects of polyethylene terephthalate (PET) microplastics during anaerobic digestion of alkaline-thermal pretreated sludge. Waste Manag. 153, 376–385 (2022). https://doi.org/10.1016/j.wasman.2022.09.016
S. Miao, B.H. Shanks, Mechanism of acetic acid esterification over sulfonic acid-functionalized mesoporous silica. J. Catal. 279(1), 136–143 (2011). https://doi.org/10.1016/j.jcat.2011.01.008
R.A. Ahmed, S. Rashid, K. Huddersman, Esterification of stearic acid using novel protonated and crosslinked amidoximated polyacrylonitrile ion exchange fibres. J. Ind. Eng. Chem. 119, 550–573 (2023). https://doi.org/10.1016/j.jiec.2022.12.001
F. Barbir, Fuel Cell Stack Design Principles with Some Design Concepts of Micro-Mini Fuel Cells (Springer, Netherlands, 2008), pp.27–46. https://doi.org/10.1007/978-1-4020-8295-5_3
S. Kakaç, A. Pramuanjaroenkij, L. Vasiliev, Mini-Micro Fuel Cells: Fundamentals and Applications (Springer, Berlin, 2008)
J. Mergel, D.L. Fritz, M. Carmo, Stack technology for PEM electrolysis, in Hydrogen Science and Engineering: Materials, Processes, Systems and Technology (Wiley‐VCH Verlag GmbH & Co. KGaA, 2016), pp. 331–358. https://doi.org/10.1002/9783527674268.ch15
E. Zhao, Y. Zhang, J. Zhan, G. Xia, G. Yu et al., Optimization and scaling-up of porous solid electrolyte electrochemical reactors for hydrogen peroxide electrosynthesis. Nat. Commun. 16(1), 3212 (2025). https://doi.org/10.1038/s41467-025-58385-2
C.-H. Chen, S.-P. Jung, S.-C. Yen, Flow distribution in the manifold of PEM fuel cell stack. J. Power. Sources 173(1), 249–263 (2007). https://doi.org/10.1016/j.jpowsour.2007.05.007