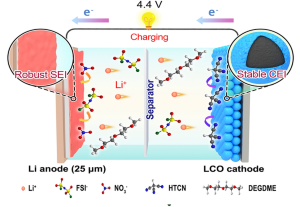
Boosting High-Voltage Practical Lithium Metal Batteries with Tailored Additives
Corresponding Author: Jun‑Tao Li
Nano-Micro Letters,
Vol. 16 (2024), Article Number: 257
Abstract
The lithium (Li) metal anode is widely regarded as an ideal anode material for high-energy-density batteries. However, uncontrolled Li dendrite growth often leads to unfavorable interfaces and low Coulombic efficiency (CE), limiting its broader application. Herein, an ether-based electrolyte (termed FGN-182) is formulated, exhibiting ultra-stable Li metal anodes through the incorporation of LiFSI and LiNO3 as dual salts. The synergistic effect of the dual salts facilitates the formation of a highly robust SEI film with fast Li+ transport kinetics. Notably, Li||Cu half cells exhibit an average CE reaching up to 99.56%. In particular, pouch cells equipped with high-loading lithium cobalt oxide (LCO, 3 mAh cm−2) cathodes, ultrathin Li chips (25 μm), and lean electrolytes (5 g Ah−1) demonstrate outstanding cycling performance, retaining 80% capacity after 125 cycles. To address the gas issue in the cathode under high voltage, cathode additives 1,3,6-tricyanohexane is incorporated with FGN-182; the resulting high-voltage LCO||Li (4.4 V) pouch cells can cycle steadily over 93 cycles. This study demonstrates that, even with the use of ether-based electrolytes, it is possible to simultaneously achieve significant improvements in both high Li utilization and electrolyte tolerance to high voltage by exploring appropriate functional additives for both the cathode and anode.
Highlights:
1 FGN-182 electrolytes exhibit highly reversible Li plating/stripping with an average Coulombic efficiency reaching up to 99.56% determined from Auerbach’s test.
2 The gas-evolution process of LiNO3 in high-voltage lithium cobalt oxide (LCO) cathodes is revealed by in situ differential electrochemical mass spectrometry.
3 Pouch cells equipped with high-loading LCO (3 mAh cm−2) cathodes, ultrathin Li chips (25 μm), and lean electrolytes (5 g Ah−1) using optimized electrolyte (FGN-182 + 1%HTCN) demonstrate outstanding cycling performance.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Y. Liang, C. Zhao, H. Yuan, Y. Chen, W. Zhang et al., A review of rechargeable batteries for portable electronic devices. InfoMat 1, 6–32 (2019). https://doi.org/10.1002/inf2.12000
- E.J. Cairns, P. Albertus, Batteries for electric and hybrid-electric vehicles. Annu. Rev. Chem. Biomol. 1, 299–320 (2010). https://doi.org/10.1146/annurev-chembioeng-073009-100942
- S.S. Rangarajan, S.P. Sunddararaj, A. Sudhakar, C.K. Shiva, U. Subramaniam et al., Lithium-ion batteries—the crux of electric vehicles with opportunities and challenges. Clean Technol. 4, 908–930 (2022). https://doi.org/10.3390/cleantechnol4040056
- Y. Gao, Z. Pan, J. Sun, Z. Liu, J. Wang, High-energy batteries: beyond lithium-ion and their long road to commercialisation. Nano-Micro Lett. 14, 94 (2022). https://doi.org/10.1007/s40820-022-00844-2
- G.E. Blomgren, The development and future of lithium ion batteries. J. Electrochem. Soc. 164, A5019 (2016). https://doi.org/10.1149/2.0251701jes
- N. Nitta, F. Wu, J.T. Lee, G. Yushin, Li-ion battery materials: present and future. Mater. Today 18, 252–264 (2015). https://doi.org/10.1016/j.mattod.2014.10.040
- J. Piątek, S. Afyon, T.M. Budnyak, S. Budnyk, M.H. Sipponen et al., Sustainable Li-ion batteries: chemistry and recycling. Adv. Energy Mater. 11, 2003456 (2021). https://doi.org/10.1002/aenm.202003456
- Z. Luo, X. Qiu, C. Liu, S. Li, C. Wang et al., Interfacial challenges towards stable Li metal anode. Nano Energy 79, 105507 (2021). https://doi.org/10.1016/j.nanoen.2020.105507
- Y. Zhang, T.-T. Zuo, J. Popovic, K. Lim, Y.-X. Yin et al., Towards better Li metal anodes: challenges and strategies. Mater. Today 33, 56–74 (2020). https://doi.org/10.1016/j.mattod.2019.09.018
- D.-H. Liu, Z. Bai, M. Li, A. Yu, D. Luo et al., Developing high safety Li-metal anodes for future high-energy Li-metal batteries: strategies and perspectives. Chem. Soc. Rev. 49, 54075445 (2020). https://doi.org/10.1039/C9CS00636B
- R. Wang, W. Cui, F. Chu, F. Wu, Lithium metal anodes: present and future. J. Energy Chem. 48, 145–159 (2020). https://doi.org/10.1016/j.jechem.2019.12.024
- Z. Sun, J. Yang, H. Xu, C. Jiang, Y. Niu et al., Enabling an inorganic-rich interface via cationic surfactant for high-performance lithium metal batteries. Nano-Micro Lett. 16, 141 (2024). https://doi.org/10.1007/s40820-024-01364-x
- S. Zhang, G. Yang, Z. Liu, X. Li, X. Wang et al., Competitive solvation enhanced stability of lithium metal anode in dual-salt electrolyte. Nano Lett. 21, 3310–3317 (2021). https://doi.org/10.1021/acs.nanolett.1c00848
- X. Zheng, L. Huang, W. Luo, H. Wang, Y. Dai et al., Tailoring electrolyte solvation chemistry toward an inorganic-rich solid-electrolyte interphase at a Li metal anode. ACS Energy Lett. 6, 2054–2063 (2021). https://doi.org/10.1021/acsenergylett.1c00647
- W. Chen, C. Zhao, B. Li, Q. Jin, X. Zhang et al., A mixed ether electrolyte for lithium metal anode protection in working lithium-sulfur batteries. Energy Envron. Mater. 3, 160–165 (2020). https://doi.org/10.1002/eem2.12073
- T. Li, X. Zhang, N. Yao, Y. Yao, L. Hou et al., Stable anion-derived solid electrolyte interphase in lithium metal batteries. Angew. Chem. Int. Ed. Engl. 60, 22683–22687 (2021). https://doi.org/10.1002/anie.202107732
- J. You, S. Zhang, L. Deng, M. Li, X. Zheng et al., Suppressing Li dendrite by a protective biopolymeric film from tamarind seed polysaccharide for high-performance Li metal anode. Electrochim. Acta 299, 636–644 (2019). https://doi.org/10.1016/j.electacta.2019.01.045
- S. Huang, K. Long, Y. Chen, T. Naren, P. Qing et al., In situ formed tribofilms as efficient organic/inorganic hybrid interlayers for stabilizing lithium metal anodes. Nano-Micro Lett. 15, 235 (2023). https://doi.org/10.1007/s40820-023-01210-6
- J. You, H. Deng, X. Zheng, H. Yan, L. Deng et al., Stabilized and almost dendrite-free Li metal anodes by in situ construction of a composite protective layer for Li metal batteries. ACS Appl. Mater. Interfaces 14, 5298–5307 (2022). https://doi.org/10.1021/acsami.1c20826
- C. Chen, Q. Liang, G. Wang, D. Liu, X. Xiong, Grain-boundary-rich artificial SEI layer for high-rate lithium metal anodes. Adv. Funct. Mater. 32, 2107249 (2022). https://doi.org/10.1002/adfm.202107249
- J. You, Y. Hu, X. Han, L. Deng, X. Zheng et al., Highly ion-conducting protective layers with nanomicro engineering for high-performance lithium metal anodes. ACS. Sustain. Chem. Eng. 11, 13407–13414 (2023). https://doi.org/10.1021/acssuschemeng.3c03017
- S. Zhang, J. You, J. Chen, Y. Hu, C. Wang et al., Aluminum-based metal-organic frameworks derived Al2O3-loading mesoporous carbon as a host matrix for lithium-metal anodes. ACS Appl. Mater. Interfaces 11, 47939–47947 (2019). https://doi.org/10.1021/acsami.9b16363
- S. Zhang, J. You, Z. He, J. Zhong, P. Zhang et al., Scalable lithiophilic/sodiophilic porous buffer layer fabrication enables uniform nucleation and growth for lithium/sodium metal batteries. Adv. Funct. Mater. 32, 2200967 (2022). https://doi.org/10.1002/adfm.202200967
- J. He, A. Manthiram, 3D CoSe@C aerogel as a host for dendrite-free lithium-metal anode and efficient sulfur cathode in Li-S full cells. Adv. Energy Mater. 10, 2002654 (2020). https://doi.org/10.1002/aenm.202002654
- C. Lu, M. Tian, X. Zheng, C. Wei, M. Rummeli et al., Cotton pad derived 3D lithiophilic carbon host for robust Li metal anode: in-situ generated ionic conductive Li3N protective decoration. J. Chem. Eng. 430, 132722 (2022). https://doi.org/10.1016/j.cej.2021.132722
- H. Lin, Z. Zhang, Y. Wang, X. Zhang, Z. Tie et al., Template-sacrificed hot fusion construction and nanoseed modification of 3D porous copper nanoscaffold host for stable-cycling lithium metal anodes. Adv. Funct. Mater. 31, 2102735 (2021). https://doi.org/10.1002/adfm.202102735
- Y. Zhang, M. Yao, T. Wang, H. Wu, Y. Zhang, A 3D hierarchical host with gradient-distributed dielectric properties toward dendrite-free lithium metal anode. Angew. Chem. Int. Ed. Engl. 63, e202403399 (2024). https://doi.org/10.1002/anie.202403399
- Z. Zhang, J. You, S. Zhang, C. Wang, Y. Zhou et al., Metal organic framework nanorod doped solid polymer electrolyte with decreased crystallinity for high-performance all-solid-state lithium batteries. ChemElectroChem 7, 1125–1134 (2020). https://doi.org/10.1002/celc.201901987
- Y. Mu, S. Yu, Y. Chen, Y. Chu, B. Wu et al., Highly efficient aligned ion-conducting network and interface chemistries for depolarized all-solid-state lithium metal batteries. Nano-Micro Lett. 16, 86 (2024). https://doi.org/10.1007/s40820-023-01301-4
- X. Li, Y. Wang, K. Xi, W. Yu, J. Feng et al., Quasi-solid-state ion-conducting arrays composite electrolytes with fast ion transport vertical-aligned interfaces for all-weather practical lithium-metal batteries. Nano-Micro Lett. 14, 210 (2022). https://doi.org/10.1007/s40820-022-00952-z
- X. Yang, J. Liu, N. Pei, Z. Chen, R. Li et al., The critical role of fillers in composite polymer electrolytes for lithium battery. Nano-Micro Lett. 15, 74 (2023). https://doi.org/10.1007/s40820-023-01051-3
- K. Wang, Y. Chen, L. Zhang, Q. Zhang, Z. Cheng et al., One step hot-pressing method for hybrid Li metal anode of solid-state lithium metal batteries. J. Mater. Sci. Technol. 153, 32–40 (2023). https://doi.org/10.1016/j.jmst.2022.12.055
- T. Krauskopf, B. Mogwitz, H. Hartmann, D.K. Singh, W.G. Zeier et al., The fast charge transfer kinetics of the lithium metal anode on the garnet-type solid electrolyte Li6.25Al0.25La3Zr2O12. Adv. Energy Mater. 10, 2000945 (2020). https://doi.org/10.1002/aenm.202000945
- X. Wang, X. Zhang, P. Shi, L. Hou, M. Zhou et al., Glycolide additives enrich organic components in the solid electrolyte interphase enabling stable ultrathin lithium metal anodes. Mater. Chem. Front. 5, 2791–2797 (2021). https://doi.org/10.1039/D0QM01134G
- J. Zhou, B. Hao, M. Peng, L. Zhang, H. Ji et al., Nonafluorobutane-1-sulfonic acid induced local high concentration additive interface for robust SEI formation of high-voltage (5 V-class) lithium metal batteries. Adv. Energy Mater. 13, 2204174 (2023). https://doi.org/10.1002/aenm.202204174
- M. Li, C. Chen, H. Luo, Q. Xu, K. Yan et al., Constructing inorganic-rich solid electrolyte interphase via adjusting electrolyte additives for stable Li metal anodes. J. Mater. Chem. A 12, 10072–10080 (2024). https://doi.org/10.1039/D3TA07655E
- A. Andersen, N.N. Rajput, K.S. Han, H. Pan, N. Govind et al., Structure and dynamics of polysulfide clusters in a nonaqueous solvent mixture of 1, 3-dioxolane and 1, 2-dimethoxyethane. Chem. Mater. 31, 2308–2319 (2019). https://doi.org/10.1021/acs.chemmater.8b03944
- P. Zhang, H. Jin, T. Wang, M. Wang, Insight into the effect of lithium-dendrite suppression by lithium bis(fluorosulfony)imide/1, 2-dimethoxyethane electrolytes. Electrochim. Acta 277, 116–126 (2018). https://doi.org/10.1016/j.electacta.2018.05.002
- G.A. Giffin, The role of concentration in electrolyte solutions for non-aqueous lithium-based batteries. Nat. Commun. 13, 5250 (2022). https://doi.org/10.1038/s41467-022-32794-z
- B.D. Adams, J. Zheng, X. Ren, W. Xu, J. Zhang, Accurate determination of coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 8, 1702097 (2018). https://doi.org/10.1002/aenm.201702097
- T. Chen, J. You, R. Li, H. Li, Y. Wang et al., Ultra-low concentration electrolyte enabling LiF-rich SEI and dense plating/stripping processes for lithium metal batteries. Adv. Sci. 9, 2203216 (2022). https://doi.org/10.1002/advs.202203216
- J. Fu, X. Ji, J. Chen, L. Chen, X. Fan et al., Lithium nitrate regulated sulfone electrolytes for lithium metal batteries. Angew. Chem. Int. Ed. Engl. 132, 22378–22385 (2020). https://doi.org/10.1002/anie.202009575
- X. Chen, Y. Yao, C. Yan, R. Zhang, X. Cheng et al., A diffusion-reaction competition mechanism to tailor lithium deposition for lithium-metal batteries. Angew. Chem. Int. Ed. Engl. 59, 7743–7747 (2020). https://doi.org/10.1002/anie.202000375
- Z. Wang, F. Qi, L. Yin, Y. Shi, C. Sun et al., An anion-tuned solid electrolyte interphase with fast ion transfer kinetics for stable lithium anodes. Adv. Energy Mater. 10, 1903843 (2020). https://doi.org/10.1002/aenm.201903843
- K.-E. Kim, J.Y. Jang, I. Park, M.-H. Woo, M.-H. Jeong et al., A combination of lithium difluorophosphate and vinylene carbonate as reducible additives to improve cycling performance of graphite electrodes at high rates. Electrochem. Commun. 61, 121–124 (2015). https://doi.org/10.1016/j.elecom.2015.10.013
- S.-J. Zhang, Z.-Y. Yin, Z.-Y. Wu, D. Luo, Y.-Y. Hu et al., Achievement of high-cyclability and high-voltage Li-metal batteries by heterogeneous SEI film with internal ionic conductivity/external electronic insulativity hybrid structure. Energy Storage Mater. 40, 337–346 (2021). https://doi.org/10.1016/j.ensm.2021.05.029
- C. Yan, Y.-X. Yao, X. Chen, X.-B. Cheng, X.-Q. Zhang et al., Lithium nitrate solvation chemistry in carbonate electrolyte sustains high-voltage lithium metal batteries. Angew. Chem. Int. Ed. 57, 14055–14059 (2018). https://doi.org/10.1002/anie.201807034
- J. Zheng, P. Yan, D. Mei, M.H. Engelhard, S.S. Cartmell et al., Highly stable operation of lithium metal batteries enabled by the formation of a transient high-concentration electrolyte layer. Adv. Energy Mater. 6, 1502151 (2016). https://doi.org/10.1002/aenm.201502151
- W.D. Richards, L.J. Miara, Y. Wang, J.C. Kim, G. Ceder, Interface stability in solid-state batteries. Chem. Mater. 28, 266–273 (2016). https://doi.org/10.1021/acs.chemmater.5b04082
- Y. Ozhabes, D. Gunceler, T. Arias, Stability and surface diffusion at lithium-electrolyte interphases with connections to dendrite suppression (2015), Preprint at arXiv:1504.05799. https://doi.org/10.48550/arXiv.1504.05799
- F. Li, J. He, J. Liu, M. Wu, Y. Hou et al., Gradient solid electrolyte interphase and lithium-ion solvation regulated by bisfluoroacetamide for stable lithium metal batteries. Angew. Chem. Int. Ed. 60, 6600–6608 (2021). https://doi.org/10.1002/anie.202013993
- Y. Liang, W. Wu, D. Li, H. Wu, C. Gao et al., Highly stable lithium metal batteries by regulating the lithium nitrate chemistry with a modified eutectic electrolyte. Adv. Energy Mater. 12, 2202493 (2022). https://doi.org/10.1002/aenm.202202493
- H. Xu, Y. Li, A. Zhou, N. Wu, S. Xin et al., Li3N-modified garnet electrolyte for all-solid-state lithium metal batteries operated at 40 ℃. Nano Lett. 18, 7414–7418 (2018). https://doi.org/10.1021/acs.nanolett.8b03902
- T. Broder, D. Silvester, L. Aldous, C. Hardacre, A. Crossley et al., The electrochemical oxidation and reduction of nitrate ions in the room temperature ionic liquid [C2mim][NTf2]; the latter behaves as a ‘melt’ rather than an ‘organic solvent.’ New J. Chem. 31, 966–972 (2007). https://doi.org/10.1039/B701097D
- X. Yang, M. Lin, G. Zheng, J. Wu, X. Wang et al., Enabling stable high-voltage LiCoO2 operation by using synergetic interfacial modification strategy. Adv. Funct. Mater. 30, 2004664 (2020). https://doi.org/10.1002/adfm.202004664
- L. Feng, Z. Yin, C. Wang, Z. Li, S. Zhang et al., Glassy/ceramic Li2TiO3/LixByOz analogous “solid electrolyte interphase” to boost 4.5 V LiCoO2 in sulfide-based all-solid-state batteries. Adv. Funct. Mater. 33, 2210744 (2023). https://doi.org/10.1002/adfm.202210744
References
Y. Liang, C. Zhao, H. Yuan, Y. Chen, W. Zhang et al., A review of rechargeable batteries for portable electronic devices. InfoMat 1, 6–32 (2019). https://doi.org/10.1002/inf2.12000
E.J. Cairns, P. Albertus, Batteries for electric and hybrid-electric vehicles. Annu. Rev. Chem. Biomol. 1, 299–320 (2010). https://doi.org/10.1146/annurev-chembioeng-073009-100942
S.S. Rangarajan, S.P. Sunddararaj, A. Sudhakar, C.K. Shiva, U. Subramaniam et al., Lithium-ion batteries—the crux of electric vehicles with opportunities and challenges. Clean Technol. 4, 908–930 (2022). https://doi.org/10.3390/cleantechnol4040056
Y. Gao, Z. Pan, J. Sun, Z. Liu, J. Wang, High-energy batteries: beyond lithium-ion and their long road to commercialisation. Nano-Micro Lett. 14, 94 (2022). https://doi.org/10.1007/s40820-022-00844-2
G.E. Blomgren, The development and future of lithium ion batteries. J. Electrochem. Soc. 164, A5019 (2016). https://doi.org/10.1149/2.0251701jes
N. Nitta, F. Wu, J.T. Lee, G. Yushin, Li-ion battery materials: present and future. Mater. Today 18, 252–264 (2015). https://doi.org/10.1016/j.mattod.2014.10.040
J. Piątek, S. Afyon, T.M. Budnyak, S. Budnyk, M.H. Sipponen et al., Sustainable Li-ion batteries: chemistry and recycling. Adv. Energy Mater. 11, 2003456 (2021). https://doi.org/10.1002/aenm.202003456
Z. Luo, X. Qiu, C. Liu, S. Li, C. Wang et al., Interfacial challenges towards stable Li metal anode. Nano Energy 79, 105507 (2021). https://doi.org/10.1016/j.nanoen.2020.105507
Y. Zhang, T.-T. Zuo, J. Popovic, K. Lim, Y.-X. Yin et al., Towards better Li metal anodes: challenges and strategies. Mater. Today 33, 56–74 (2020). https://doi.org/10.1016/j.mattod.2019.09.018
D.-H. Liu, Z. Bai, M. Li, A. Yu, D. Luo et al., Developing high safety Li-metal anodes for future high-energy Li-metal batteries: strategies and perspectives. Chem. Soc. Rev. 49, 54075445 (2020). https://doi.org/10.1039/C9CS00636B
R. Wang, W. Cui, F. Chu, F. Wu, Lithium metal anodes: present and future. J. Energy Chem. 48, 145–159 (2020). https://doi.org/10.1016/j.jechem.2019.12.024
Z. Sun, J. Yang, H. Xu, C. Jiang, Y. Niu et al., Enabling an inorganic-rich interface via cationic surfactant for high-performance lithium metal batteries. Nano-Micro Lett. 16, 141 (2024). https://doi.org/10.1007/s40820-024-01364-x
S. Zhang, G. Yang, Z. Liu, X. Li, X. Wang et al., Competitive solvation enhanced stability of lithium metal anode in dual-salt electrolyte. Nano Lett. 21, 3310–3317 (2021). https://doi.org/10.1021/acs.nanolett.1c00848
X. Zheng, L. Huang, W. Luo, H. Wang, Y. Dai et al., Tailoring electrolyte solvation chemistry toward an inorganic-rich solid-electrolyte interphase at a Li metal anode. ACS Energy Lett. 6, 2054–2063 (2021). https://doi.org/10.1021/acsenergylett.1c00647
W. Chen, C. Zhao, B. Li, Q. Jin, X. Zhang et al., A mixed ether electrolyte for lithium metal anode protection in working lithium-sulfur batteries. Energy Envron. Mater. 3, 160–165 (2020). https://doi.org/10.1002/eem2.12073
T. Li, X. Zhang, N. Yao, Y. Yao, L. Hou et al., Stable anion-derived solid electrolyte interphase in lithium metal batteries. Angew. Chem. Int. Ed. Engl. 60, 22683–22687 (2021). https://doi.org/10.1002/anie.202107732
J. You, S. Zhang, L. Deng, M. Li, X. Zheng et al., Suppressing Li dendrite by a protective biopolymeric film from tamarind seed polysaccharide for high-performance Li metal anode. Electrochim. Acta 299, 636–644 (2019). https://doi.org/10.1016/j.electacta.2019.01.045
S. Huang, K. Long, Y. Chen, T. Naren, P. Qing et al., In situ formed tribofilms as efficient organic/inorganic hybrid interlayers for stabilizing lithium metal anodes. Nano-Micro Lett. 15, 235 (2023). https://doi.org/10.1007/s40820-023-01210-6
J. You, H. Deng, X. Zheng, H. Yan, L. Deng et al., Stabilized and almost dendrite-free Li metal anodes by in situ construction of a composite protective layer for Li metal batteries. ACS Appl. Mater. Interfaces 14, 5298–5307 (2022). https://doi.org/10.1021/acsami.1c20826
C. Chen, Q. Liang, G. Wang, D. Liu, X. Xiong, Grain-boundary-rich artificial SEI layer for high-rate lithium metal anodes. Adv. Funct. Mater. 32, 2107249 (2022). https://doi.org/10.1002/adfm.202107249
J. You, Y. Hu, X. Han, L. Deng, X. Zheng et al., Highly ion-conducting protective layers with nanomicro engineering for high-performance lithium metal anodes. ACS. Sustain. Chem. Eng. 11, 13407–13414 (2023). https://doi.org/10.1021/acssuschemeng.3c03017
S. Zhang, J. You, J. Chen, Y. Hu, C. Wang et al., Aluminum-based metal-organic frameworks derived Al2O3-loading mesoporous carbon as a host matrix for lithium-metal anodes. ACS Appl. Mater. Interfaces 11, 47939–47947 (2019). https://doi.org/10.1021/acsami.9b16363
S. Zhang, J. You, Z. He, J. Zhong, P. Zhang et al., Scalable lithiophilic/sodiophilic porous buffer layer fabrication enables uniform nucleation and growth for lithium/sodium metal batteries. Adv. Funct. Mater. 32, 2200967 (2022). https://doi.org/10.1002/adfm.202200967
J. He, A. Manthiram, 3D CoSe@C aerogel as a host for dendrite-free lithium-metal anode and efficient sulfur cathode in Li-S full cells. Adv. Energy Mater. 10, 2002654 (2020). https://doi.org/10.1002/aenm.202002654
C. Lu, M. Tian, X. Zheng, C. Wei, M. Rummeli et al., Cotton pad derived 3D lithiophilic carbon host for robust Li metal anode: in-situ generated ionic conductive Li3N protective decoration. J. Chem. Eng. 430, 132722 (2022). https://doi.org/10.1016/j.cej.2021.132722
H. Lin, Z. Zhang, Y. Wang, X. Zhang, Z. Tie et al., Template-sacrificed hot fusion construction and nanoseed modification of 3D porous copper nanoscaffold host for stable-cycling lithium metal anodes. Adv. Funct. Mater. 31, 2102735 (2021). https://doi.org/10.1002/adfm.202102735
Y. Zhang, M. Yao, T. Wang, H. Wu, Y. Zhang, A 3D hierarchical host with gradient-distributed dielectric properties toward dendrite-free lithium metal anode. Angew. Chem. Int. Ed. Engl. 63, e202403399 (2024). https://doi.org/10.1002/anie.202403399
Z. Zhang, J. You, S. Zhang, C. Wang, Y. Zhou et al., Metal organic framework nanorod doped solid polymer electrolyte with decreased crystallinity for high-performance all-solid-state lithium batteries. ChemElectroChem 7, 1125–1134 (2020). https://doi.org/10.1002/celc.201901987
Y. Mu, S. Yu, Y. Chen, Y. Chu, B. Wu et al., Highly efficient aligned ion-conducting network and interface chemistries for depolarized all-solid-state lithium metal batteries. Nano-Micro Lett. 16, 86 (2024). https://doi.org/10.1007/s40820-023-01301-4
X. Li, Y. Wang, K. Xi, W. Yu, J. Feng et al., Quasi-solid-state ion-conducting arrays composite electrolytes with fast ion transport vertical-aligned interfaces for all-weather practical lithium-metal batteries. Nano-Micro Lett. 14, 210 (2022). https://doi.org/10.1007/s40820-022-00952-z
X. Yang, J. Liu, N. Pei, Z. Chen, R. Li et al., The critical role of fillers in composite polymer electrolytes for lithium battery. Nano-Micro Lett. 15, 74 (2023). https://doi.org/10.1007/s40820-023-01051-3
K. Wang, Y. Chen, L. Zhang, Q. Zhang, Z. Cheng et al., One step hot-pressing method for hybrid Li metal anode of solid-state lithium metal batteries. J. Mater. Sci. Technol. 153, 32–40 (2023). https://doi.org/10.1016/j.jmst.2022.12.055
T. Krauskopf, B. Mogwitz, H. Hartmann, D.K. Singh, W.G. Zeier et al., The fast charge transfer kinetics of the lithium metal anode on the garnet-type solid electrolyte Li6.25Al0.25La3Zr2O12. Adv. Energy Mater. 10, 2000945 (2020). https://doi.org/10.1002/aenm.202000945
X. Wang, X. Zhang, P. Shi, L. Hou, M. Zhou et al., Glycolide additives enrich organic components in the solid electrolyte interphase enabling stable ultrathin lithium metal anodes. Mater. Chem. Front. 5, 2791–2797 (2021). https://doi.org/10.1039/D0QM01134G
J. Zhou, B. Hao, M. Peng, L. Zhang, H. Ji et al., Nonafluorobutane-1-sulfonic acid induced local high concentration additive interface for robust SEI formation of high-voltage (5 V-class) lithium metal batteries. Adv. Energy Mater. 13, 2204174 (2023). https://doi.org/10.1002/aenm.202204174
M. Li, C. Chen, H. Luo, Q. Xu, K. Yan et al., Constructing inorganic-rich solid electrolyte interphase via adjusting electrolyte additives for stable Li metal anodes. J. Mater. Chem. A 12, 10072–10080 (2024). https://doi.org/10.1039/D3TA07655E
A. Andersen, N.N. Rajput, K.S. Han, H. Pan, N. Govind et al., Structure and dynamics of polysulfide clusters in a nonaqueous solvent mixture of 1, 3-dioxolane and 1, 2-dimethoxyethane. Chem. Mater. 31, 2308–2319 (2019). https://doi.org/10.1021/acs.chemmater.8b03944
P. Zhang, H. Jin, T. Wang, M. Wang, Insight into the effect of lithium-dendrite suppression by lithium bis(fluorosulfony)imide/1, 2-dimethoxyethane electrolytes. Electrochim. Acta 277, 116–126 (2018). https://doi.org/10.1016/j.electacta.2018.05.002
G.A. Giffin, The role of concentration in electrolyte solutions for non-aqueous lithium-based batteries. Nat. Commun. 13, 5250 (2022). https://doi.org/10.1038/s41467-022-32794-z
B.D. Adams, J. Zheng, X. Ren, W. Xu, J. Zhang, Accurate determination of coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 8, 1702097 (2018). https://doi.org/10.1002/aenm.201702097
T. Chen, J. You, R. Li, H. Li, Y. Wang et al., Ultra-low concentration electrolyte enabling LiF-rich SEI and dense plating/stripping processes for lithium metal batteries. Adv. Sci. 9, 2203216 (2022). https://doi.org/10.1002/advs.202203216
J. Fu, X. Ji, J. Chen, L. Chen, X. Fan et al., Lithium nitrate regulated sulfone electrolytes for lithium metal batteries. Angew. Chem. Int. Ed. Engl. 132, 22378–22385 (2020). https://doi.org/10.1002/anie.202009575
X. Chen, Y. Yao, C. Yan, R. Zhang, X. Cheng et al., A diffusion-reaction competition mechanism to tailor lithium deposition for lithium-metal batteries. Angew. Chem. Int. Ed. Engl. 59, 7743–7747 (2020). https://doi.org/10.1002/anie.202000375
Z. Wang, F. Qi, L. Yin, Y. Shi, C. Sun et al., An anion-tuned solid electrolyte interphase with fast ion transfer kinetics for stable lithium anodes. Adv. Energy Mater. 10, 1903843 (2020). https://doi.org/10.1002/aenm.201903843
K.-E. Kim, J.Y. Jang, I. Park, M.-H. Woo, M.-H. Jeong et al., A combination of lithium difluorophosphate and vinylene carbonate as reducible additives to improve cycling performance of graphite electrodes at high rates. Electrochem. Commun. 61, 121–124 (2015). https://doi.org/10.1016/j.elecom.2015.10.013
S.-J. Zhang, Z.-Y. Yin, Z.-Y. Wu, D. Luo, Y.-Y. Hu et al., Achievement of high-cyclability and high-voltage Li-metal batteries by heterogeneous SEI film with internal ionic conductivity/external electronic insulativity hybrid structure. Energy Storage Mater. 40, 337–346 (2021). https://doi.org/10.1016/j.ensm.2021.05.029
C. Yan, Y.-X. Yao, X. Chen, X.-B. Cheng, X.-Q. Zhang et al., Lithium nitrate solvation chemistry in carbonate electrolyte sustains high-voltage lithium metal batteries. Angew. Chem. Int. Ed. 57, 14055–14059 (2018). https://doi.org/10.1002/anie.201807034
J. Zheng, P. Yan, D. Mei, M.H. Engelhard, S.S. Cartmell et al., Highly stable operation of lithium metal batteries enabled by the formation of a transient high-concentration electrolyte layer. Adv. Energy Mater. 6, 1502151 (2016). https://doi.org/10.1002/aenm.201502151
W.D. Richards, L.J. Miara, Y. Wang, J.C. Kim, G. Ceder, Interface stability in solid-state batteries. Chem. Mater. 28, 266–273 (2016). https://doi.org/10.1021/acs.chemmater.5b04082
Y. Ozhabes, D. Gunceler, T. Arias, Stability and surface diffusion at lithium-electrolyte interphases with connections to dendrite suppression (2015), Preprint at arXiv:1504.05799. https://doi.org/10.48550/arXiv.1504.05799
F. Li, J. He, J. Liu, M. Wu, Y. Hou et al., Gradient solid electrolyte interphase and lithium-ion solvation regulated by bisfluoroacetamide for stable lithium metal batteries. Angew. Chem. Int. Ed. 60, 6600–6608 (2021). https://doi.org/10.1002/anie.202013993
Y. Liang, W. Wu, D. Li, H. Wu, C. Gao et al., Highly stable lithium metal batteries by regulating the lithium nitrate chemistry with a modified eutectic electrolyte. Adv. Energy Mater. 12, 2202493 (2022). https://doi.org/10.1002/aenm.202202493
H. Xu, Y. Li, A. Zhou, N. Wu, S. Xin et al., Li3N-modified garnet electrolyte for all-solid-state lithium metal batteries operated at 40 ℃. Nano Lett. 18, 7414–7418 (2018). https://doi.org/10.1021/acs.nanolett.8b03902
T. Broder, D. Silvester, L. Aldous, C. Hardacre, A. Crossley et al., The electrochemical oxidation and reduction of nitrate ions in the room temperature ionic liquid [C2mim][NTf2]; the latter behaves as a ‘melt’ rather than an ‘organic solvent.’ New J. Chem. 31, 966–972 (2007). https://doi.org/10.1039/B701097D
X. Yang, M. Lin, G. Zheng, J. Wu, X. Wang et al., Enabling stable high-voltage LiCoO2 operation by using synergetic interfacial modification strategy. Adv. Funct. Mater. 30, 2004664 (2020). https://doi.org/10.1002/adfm.202004664
L. Feng, Z. Yin, C. Wang, Z. Li, S. Zhang et al., Glassy/ceramic Li2TiO3/LixByOz analogous “solid electrolyte interphase” to boost 4.5 V LiCoO2 in sulfide-based all-solid-state batteries. Adv. Funct. Mater. 33, 2210744 (2023). https://doi.org/10.1002/adfm.202210744