Ligand Exchange of Colloidal ZnO Nanocrystals from the High Temperature and Nonaqueous Approach
Corresponding Author: Yizheng Jin
Nano-Micro Letters,
Vol. 5 No. 4 (2013), Article Number: 274-280
Abstract
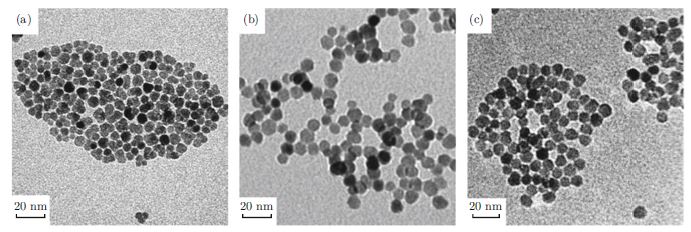
Colloidal zinc oxide (ZnO) nanocrystals generated from the high temperature and nonaqueous approache are attractive for use in solution-processed electrical and optoelectronic devices. However, the as-prepared colloidal ZnO nanocrystals by this approach are generally capped by ligands with long alkyl-chains, which is disadvantage for solution-processed devices due to hindering charge transport. Here we demonstrate an effective ligand exchange process for the colloidal ZnO nanocrystals from the high temperature and nonaqueous approach by using n-butylamine. The ligand exchange process was carefully characterized. The thin films based on colloidal ZnO nanocrystals with ligand exchange exhibited dramatically enhanced UV photoconductivity.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- G. Konstantatos, I. Howard, A. Fischer, S. Hoogland, J. Clifford, E. Klem, L. Levina and E. H. Sargent, “Ultrasensitive solution-cast quantum dot photodetectors”, Nature 442(7099), 180–183 (2006). http://dx.doi.org/10.1038/nature04855
- D. V. Talapin, J. S. Lee, M. V. Kovalenko, and E. V. Shevchenko, “Prospects of colloidal nanocrystals for electronic and optoelectronic applications”, Chem. Rev. 110(1), 389–458 (2010). http://dx.doi.org/10.1021/cr900137k
- Ü. Özgür, Ya. I. Alivov, C. Liu, A. Teke, M. A. Reshchikov, S. Dogan, V. Avrutin, S. J. Cho, and H. Morkoç, “A comprehensive review of ZnO materials and devices”, J. Appl. Phys. 98(4), 041301–041403 (2005). http://dx.doi.org/10.1063/1.1992666
- B. Q. Sun and H. Sirringhaus, “Solution-processed zinc oxide field-effect transistors based on self-assembly of colloidal nanorods”, Nano Lett. 5(12), 2408–2413 (2005). http://dx.doi.org/10.1021/nl051586w
- Y. Z. Jin, J. P. Wang, B. Q. Sun, J. C. Blakesley and N. C. Greenham, “Solution-processed ultraviolet photodetectors based on colloidal zno nanoparticles”, Nano. Lett. 8(6), 1649–1653 (2008). http://dx.doi.org/10.1021/nl0803702
- W. J. E. Beek, M. M. Wienk and R. A. J. Janssen, “Efficient hybrid solar cells from zinc oxide nanoparticles and a conjugated polymer”, Adv. Mater. 16(12), 1009–1013 (2004). http://dx.doi.org/10.1002/adma.200306659
- H. M. P. Wong, P. Wang, A. Abrusci, M. Svensson, M. R. Andersson and N. C. Greenham, “Donor and acceptor behavior in a polyfluorene for photovoltaics”, J. Phys. Chem. C 111(13), 5244–5249 (2007). http://dx.doi.org/10.1021/jp068536f
- J. P. Wang, B. Q. Sun, F. Gao and N. C. Greenham, “Memristive devices based on solution-processed ZnO nanocrystals”, Phys. Status Solidi A 207(2), 484–487 (2010). http://dx.doi.org/10.1002/pssa.200925467
- J. B. You, C. C. Chen, L. T. Dou, S. Murase, H. S. Duan and S. A. Hawks, T. Xu, H. J. Son, L. P. Yu, G. Li and Y. Yang, “Metal oxide nanoparticles as an electron-transport layer in high-performance and stable inverted polymer solar cells”, Adv. Mater. 24(38), 5267–5272 (2012). http://dx.doi.org/10.1002/adma.201201958
- C. B. Murray, D. J. Noms and M. G. Bawendi, “Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites”, J. Am. Chem. Soc. 115(19), 8706–8715 (1993). http://dx.doi.org/10.1021/ja00072a025
- Y. F. Chen, M. Kim, G. D. Lian, M. B. Johnson and X. G. Peng, “Side reactions in controlling the quality, yield, and stability of high quality colloidal nanocrystals”, J. Am. Chem. Soc. 127(38), 13331–13337 (2005). http://dx.doi.org/10.1021/ja053151g
- Y. F. Yang, Y. Z. Jin, H. P. He, Q. L. Wang, Y. Tu, H. M. Lu and Z. Z. Ye, “Dopant-induced shape evolution of colloidal nanocrystals: the case of zinc oxide”, J. Am. Chem. Soc. 132(38), 13381–13394 (2010). http://dx.doi.org/10.1021/ja103956p
- X. Wang, Y. Z. Jin, H. P. He, F. Yang, Y. F. Yang and Z. Z. Ye, “Bandgap engineering and shape control of colloidal CdxZn1−xO nanocrystals”, Nanoscale 5(14), 6464–6468 (2013). http://dx.doi.org/10.1039/C3NR01124K
- R. Buonsanti, A. Llordes, S. Aloni, B. A. Helms and D. J. Milliron, “Tunable infrared absorption and visible transparency of colloidal aluminum-doped zinc oxide nanocrystals”, Nano. Lett. 11(11), 4706–4710 (2011). http://dx.doi.org/10.1021/nl203030f
- H. T. Zhang, B. Hu, L. F. Sun, R. Hovden, F. W. Wise, D. A. Muller and R. D. Robinson, “Surfactant ligand removal and rational fabrication of inorganically connected quantum dots”, Nano. Lett. 11(12), 5356–5361 (2011). http://dx.doi.org/10.1021/nl202892p
- A. Nag, M. V. Kovalenko, J. S. Lee, W. Y. Liu, B. Spokoyny and D. V. Talapin, “Metal-free inorganic ligands for colloidal nanocrystals: S2−, HS−, Se2−, HSe−, Te2−, HTe−, TeS32−, OH−, and NH2− as surface ligands”, J. Am. Chem. Soc. 133(27), 10612–10620 (2011). http://dx.doi.org/10.1021/ja2029415
- H. K. Kim, K. K. Kim, S. J. Park, T. Y. Seong and I. Adesida, “Formation of low resistance nonalloyed Al/Pt ohmic contacts on n-type ZnO epitaxial layer”, J. Appl. Phys. 94(6), 4225–4227 (2003). http://dx.doi.org/10.1063/1.1604475
- Q. Y. Qu, H. W. Geng, R. X. Peng, Q. Cui, X. H. Gu, F. Q. Li and M. T. Wang, “Chemically binding carboxylic acids onto TiO2 nanoparticles with adjustable coverage by solvothermal strategy”, Langmuir 26(12), 9539–9546 (2010). http://dx.doi.org/10.1021/la100121n
- A. Hassinen, I. Moreels, K. D. Nolf, P. F. Smet, C. Martins and Z. Hens, “Short-chain alcohols strip X-type ligands and quench the luminescence of PbSe and CdSe quantum dots, acetonitrile does not”, J. Am. Chem. Soc. 134(51), 20705–20712 (2012). http://dx.doi.org/10.1021/ja308861d
- O. Chen, Y. A. Yang, T. Wang, H. M. Wu, C. G. Niu, J. H. Yang and Y. C. Cao, “Surface-functionalization-dependent optical properties of II–VI semiconductor nanocrystals”, J. Am. Chem. Soc. 133(43), 17504–17512 (2011). http://dx.doi.org/10.1021/ja208337r
- C. Soci, A. Zhang, B. Xiang, S. A. Dayeh, D. P. R. Aplin, J. Park, X. Y. Bao, Y. H. Lo and D. Wag, “Zno nanowire UV photodetectors with high internal gain”, Nano. Lett. 7(4), 1003–1009 (2007). http://dx.doi.org/10.1021/nl070111x
References
G. Konstantatos, I. Howard, A. Fischer, S. Hoogland, J. Clifford, E. Klem, L. Levina and E. H. Sargent, “Ultrasensitive solution-cast quantum dot photodetectors”, Nature 442(7099), 180–183 (2006). http://dx.doi.org/10.1038/nature04855
D. V. Talapin, J. S. Lee, M. V. Kovalenko, and E. V. Shevchenko, “Prospects of colloidal nanocrystals for electronic and optoelectronic applications”, Chem. Rev. 110(1), 389–458 (2010). http://dx.doi.org/10.1021/cr900137k
Ü. Özgür, Ya. I. Alivov, C. Liu, A. Teke, M. A. Reshchikov, S. Dogan, V. Avrutin, S. J. Cho, and H. Morkoç, “A comprehensive review of ZnO materials and devices”, J. Appl. Phys. 98(4), 041301–041403 (2005). http://dx.doi.org/10.1063/1.1992666
B. Q. Sun and H. Sirringhaus, “Solution-processed zinc oxide field-effect transistors based on self-assembly of colloidal nanorods”, Nano Lett. 5(12), 2408–2413 (2005). http://dx.doi.org/10.1021/nl051586w
Y. Z. Jin, J. P. Wang, B. Q. Sun, J. C. Blakesley and N. C. Greenham, “Solution-processed ultraviolet photodetectors based on colloidal zno nanoparticles”, Nano. Lett. 8(6), 1649–1653 (2008). http://dx.doi.org/10.1021/nl0803702
W. J. E. Beek, M. M. Wienk and R. A. J. Janssen, “Efficient hybrid solar cells from zinc oxide nanoparticles and a conjugated polymer”, Adv. Mater. 16(12), 1009–1013 (2004). http://dx.doi.org/10.1002/adma.200306659
H. M. P. Wong, P. Wang, A. Abrusci, M. Svensson, M. R. Andersson and N. C. Greenham, “Donor and acceptor behavior in a polyfluorene for photovoltaics”, J. Phys. Chem. C 111(13), 5244–5249 (2007). http://dx.doi.org/10.1021/jp068536f
J. P. Wang, B. Q. Sun, F. Gao and N. C. Greenham, “Memristive devices based on solution-processed ZnO nanocrystals”, Phys. Status Solidi A 207(2), 484–487 (2010). http://dx.doi.org/10.1002/pssa.200925467
J. B. You, C. C. Chen, L. T. Dou, S. Murase, H. S. Duan and S. A. Hawks, T. Xu, H. J. Son, L. P. Yu, G. Li and Y. Yang, “Metal oxide nanoparticles as an electron-transport layer in high-performance and stable inverted polymer solar cells”, Adv. Mater. 24(38), 5267–5272 (2012). http://dx.doi.org/10.1002/adma.201201958
C. B. Murray, D. J. Noms and M. G. Bawendi, “Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites”, J. Am. Chem. Soc. 115(19), 8706–8715 (1993). http://dx.doi.org/10.1021/ja00072a025
Y. F. Chen, M. Kim, G. D. Lian, M. B. Johnson and X. G. Peng, “Side reactions in controlling the quality, yield, and stability of high quality colloidal nanocrystals”, J. Am. Chem. Soc. 127(38), 13331–13337 (2005). http://dx.doi.org/10.1021/ja053151g
Y. F. Yang, Y. Z. Jin, H. P. He, Q. L. Wang, Y. Tu, H. M. Lu and Z. Z. Ye, “Dopant-induced shape evolution of colloidal nanocrystals: the case of zinc oxide”, J. Am. Chem. Soc. 132(38), 13381–13394 (2010). http://dx.doi.org/10.1021/ja103956p
X. Wang, Y. Z. Jin, H. P. He, F. Yang, Y. F. Yang and Z. Z. Ye, “Bandgap engineering and shape control of colloidal CdxZn1−xO nanocrystals”, Nanoscale 5(14), 6464–6468 (2013). http://dx.doi.org/10.1039/C3NR01124K
R. Buonsanti, A. Llordes, S. Aloni, B. A. Helms and D. J. Milliron, “Tunable infrared absorption and visible transparency of colloidal aluminum-doped zinc oxide nanocrystals”, Nano. Lett. 11(11), 4706–4710 (2011). http://dx.doi.org/10.1021/nl203030f
H. T. Zhang, B. Hu, L. F. Sun, R. Hovden, F. W. Wise, D. A. Muller and R. D. Robinson, “Surfactant ligand removal and rational fabrication of inorganically connected quantum dots”, Nano. Lett. 11(12), 5356–5361 (2011). http://dx.doi.org/10.1021/nl202892p
A. Nag, M. V. Kovalenko, J. S. Lee, W. Y. Liu, B. Spokoyny and D. V. Talapin, “Metal-free inorganic ligands for colloidal nanocrystals: S2−, HS−, Se2−, HSe−, Te2−, HTe−, TeS32−, OH−, and NH2− as surface ligands”, J. Am. Chem. Soc. 133(27), 10612–10620 (2011). http://dx.doi.org/10.1021/ja2029415
H. K. Kim, K. K. Kim, S. J. Park, T. Y. Seong and I. Adesida, “Formation of low resistance nonalloyed Al/Pt ohmic contacts on n-type ZnO epitaxial layer”, J. Appl. Phys. 94(6), 4225–4227 (2003). http://dx.doi.org/10.1063/1.1604475
Q. Y. Qu, H. W. Geng, R. X. Peng, Q. Cui, X. H. Gu, F. Q. Li and M. T. Wang, “Chemically binding carboxylic acids onto TiO2 nanoparticles with adjustable coverage by solvothermal strategy”, Langmuir 26(12), 9539–9546 (2010). http://dx.doi.org/10.1021/la100121n
A. Hassinen, I. Moreels, K. D. Nolf, P. F. Smet, C. Martins and Z. Hens, “Short-chain alcohols strip X-type ligands and quench the luminescence of PbSe and CdSe quantum dots, acetonitrile does not”, J. Am. Chem. Soc. 134(51), 20705–20712 (2012). http://dx.doi.org/10.1021/ja308861d
O. Chen, Y. A. Yang, T. Wang, H. M. Wu, C. G. Niu, J. H. Yang and Y. C. Cao, “Surface-functionalization-dependent optical properties of II–VI semiconductor nanocrystals”, J. Am. Chem. Soc. 133(43), 17504–17512 (2011). http://dx.doi.org/10.1021/ja208337r
C. Soci, A. Zhang, B. Xiang, S. A. Dayeh, D. P. R. Aplin, J. Park, X. Y. Bao, Y. H. Lo and D. Wag, “Zno nanowire UV photodetectors with high internal gain”, Nano. Lett. 7(4), 1003–1009 (2007). http://dx.doi.org/10.1021/nl070111x