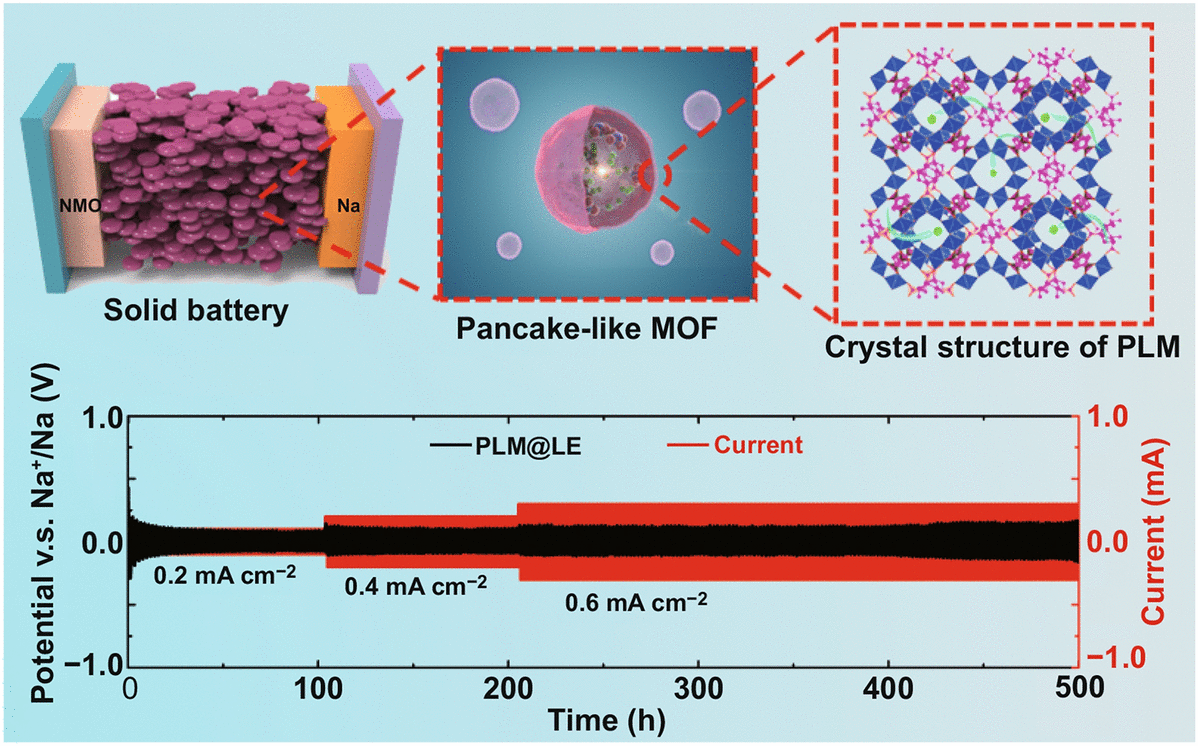
Pancake-Like MOF Solid-State Electrolytes with Fast Ion Migration for High-Performance Sodium Battery
Corresponding Author: Liqiang Mai
Nano-Micro Letters,
Vol. 13 (2021), Article Number: 105
Abstract
Solid-state electrolyte (SSE) of the sodium-ion battery have attracted tremendous attention in the next generation energy storage materials on account of their wide electrochemical window and thermal stability. However, the high interfacial impedance, low ion transference number and complex preparation process restrict the application of SSE. Herein, inspired by the excellent sieving function and high specific surface area of red blood cells, we obtained a solid-like electrolyte (SLE) based on the combination of the pancake-like metal–organic framework (MOF) with liquid electrolyte, possessing a high ionic conductivity of 6.60 × 10–4 S cm−1, and excellent sodium metal compatibility. In addition, we investigated the ion restriction effect of MOF’s apertures size and special functional groups, and the ion transference number increased from 0.16 to 0.33. Finally, the assembled Na0.44MnO2//SLE//Na full batteries showed no obvious capacity decrease after 160 cycles. This material design of SLE in our work is an important key to obtain fast ion migration SLE for high-performance sodium-ion batteries.
Highlights:
1 A pancake-like morphology solid-like eletrolyte of sodium battery with high ionic conductivity (6.60 × 10–4 S cm−1) was obtained by simple hydrothermal method.
2 Solid-like electrolyte with pancake-like morphology showed good interface contact and excellent compatibility (stable cycle over 500 h at 0.6 mA cm−2) with sodium metal.
3 Provides possible repulsive force explanation for the restriction of ion transport by MOF.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- A. Sternberg, A. Bardow, Power-to-what? Environmental assessment of energy storage systems. Energy Environ. Sci. 8(2), 389–400 (2015). https://doi.org/10.1039/c4ee03051f
- H.S. Chen, T.N. Cong, W. Yang, C.Q. Tan, Y.L. Li et al., Progress in electrical energy storage system: a critical review. Progr. Nat. Sci. 19(3), 291–312 (2009). https://doi.org/10.1016/j.pnsc.2008.07.014
- M.D. Slater, D. Kim, E. Lee, C.S. Johnson, Sodium-ion batteries. Adv. Funct. Mater. 23(8), 947–958 (2013). https://doi.org/10.1002/adfm.201200691
- H.S. Li, L.L. Peng, Y. Zhu, D.H. Chen, X.G. Zhang et al., An advanced high-energy sodium ion full battery based on nanostructured Na2Ti3O7/VOPO4 layered materials. Energy Environ. Sci. 9(11), 3399–3405 (2016). https://doi.org/10.1039/c6ee00794e
- B. Zhang, G. Rousse, D. Foix, R. Dugas, D.A.D. Corte et al., Microsized Sn as advanced anodes in glyme-based electrolyte for Na-ion batteries. Adv. Mater. 28(44), 9824–9830 (2016). https://doi.org/10.1002/adma.201603212
- X.Q. Ma, L. Zou, W.X. Zhao, Tailoring hollow microflower-shaped CoSe2 anodes in sodium ion batteries with high cycling stability. Chem. Commun. 54(74), 10507–10510 (2018). https://doi.org/10.1039/c8cc04426k
- G.L. Woods, K.L. White, D.K. Vanderwall, G.P. Li, K.I. Aston et al., A mule cloned from fetal cells by nuclear transfer. Science 301(5636), 1063 (2003). https://doi.org/10.1126/science.1086743
- H.-D. Nguyen, G.-T. Kim, J.L. Shi, E. Paillard, P. Judeinstein et al., Nanostructured multi-block copolymer single-ion conductors for safer high-performance lithium batteries. Energy Environ. Sci. 11(11), 3298–3309 (2018). https://doi.org/10.1039/c8ee02093k
- Y.X. Huang, L.Z. Zhao, L. Li, M. Xie, F. Wu et al., Electrolytes and electrolyte/electrode interfaces in sodium-ion batteries: from scientific research to practical application. Adv. Mater. 31(21), 1808393 (2019). https://doi.org/10.1002/adma.201808393
- Y. Lu, L. Li, Q. Zhang, Z.Q. Niu, J. Chen, Electrolyte and interface engineering for solid-state sodium batteries. Joule 2(9), 1747–1770 (2018). https://doi.org/10.1016/j.joule.2018.07.028
- L. Xu, S. Tang, Y. Cheng, K.Y. Wang, J.Y. Liang et al., Interfaces in solid-state lithium batteries. Joule 2(10), 1991–2015 (2018). https://doi.org/10.1016/j.joule.2018.07.009
- W.R. Hou, X.W. Guo, X.Y. Shen, K. Amine, H.J. Yu et al., Solid electrolytes and interfaces in all-solid-state sodium batteries: progress and perspective. Nano Energy 52, 279–291 (2018). https://doi.org/10.1016/j.nanoen.2018.07.036
- L. Porcarelli, A.S. Shaplov, F. Bella, J.R. Nair, D. Mecerreyes et al., Single-ion conducting polymer electrolytes for lithium metal polymer batteries that operate at ambient temperature. ACS Energy Lett. 1(4), 678–682 (2016). https://doi.org/10.1021/acsenergylett.6b00216
- H.C. Gao, L.G. Xue, S. Xin, K. Park, J.B. Goodenough, A plastic-crystal electrolyte interphase for all-solid-state sodium batteries. Angew. Chem. Int. Ed. 56(20), 5541–5545 (2017). https://doi.org/10.1002/anie.201702003
- F. Lv, Z.Y. Wang, L.Y. Shi, J.F. Zhu, K. Edström et al., Challenges and development of composite solid-state electrolytes for high-performance lithium ion batteries. J. Power Sour. 441, 227175 (2019). https://doi.org/10.1016/j.jpowsour.2019.227175
- H.L. Li, M. Eddaoudi, M. O’Keeffe, O.M. Yaghi, Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nat. Energy 402(6759), 276–279 (1999). https://doi.org/10.1038/46248
- H.-C. Zhou, J.R. Long, O.M. Yaghi, Introduction to metal-organic frameworks. Chem. Rev. 112(2), 673–674 (2012). https://doi.org/10.1021/cr300014x
- M. Li, D. Li, M. O’Keeffe, O.M. Yaghi, Topological analysis of metal-organic frameworks with polytopic linkers and/or multiple building units and the minimal transitivity principle. Chem. Rev. 114(2), 1343–1370 (2014). https://doi.org/10.1021/cr400392k
- W.G. Lu, Z.W. Wei, Z.-Y. Gu, T.-F. Liu, J. Park et al., Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 43(16), 5561–5593 (2014). https://doi.org/10.1039/c4cs00003j
- R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa et al., High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 319(5865), 939–943 (2008). https://doi.org/10.1126/science.1152516
- Z.Q. Wang, R. Tan, H.B. Wang, L.Y. Yang, J.T. Hu et al., A metal-organic-framework-based electrolyte with nanowetted interfaces for high-energy-density solid-state lithium battery. Adv. Mater. 30(2), 1704436 (2018). https://doi.org/10.1002/adma.201704436
- Z.Q. Wang, J.T. Hu, L. Han, Z.J. Wang, H.B. Wang et al., A MOF-based single-ion Zn2+ solid electrolyte leading to dendrite-free rechargeable Zn batteries. Nano Energy 56, 92–99 (2019). https://doi.org/10.1016/j.nanoen.2018.11.038
- S. Ma, L. Shen, Q. Liu, W. Shi, C. Zhang et al., Class of solid-like electrolytes for rechargeable batteries based on metal-organic frameworks infiltrated with liquid electrolytes. ACS Appl. Mater. Interfaces 12(39), 43824–43832 (2020). https://doi.org/10.1021/acsami.0c13437
- S.S. Park, Y. Tulchinsky, M. Dinca, Single-ion Li+, Na+, and Mg2+ solid electrolytes supported by a mesoporous anionic Cu-azolate metal-organic framework. J. Am. Chem. Soc. 139(38), 13260–13263 (2017). https://doi.org/10.1021/jacs.7b06197
- L. Shen, H.B. Wu, F. Liu, J.L. Brosmer, G.R. Shen et al., Creating lithium-ion electrolytes with biomimetic ionic channels in metal-organic frameworks. Adv. Mater. 30(23), e1707476 (2018). https://doi.org/10.1002/adma.201707476
- Jo.E.C.J. Li, Q.Q. Liu, L.N. Gao, Y.F. Xu, X.Q. Kong et al., Quasi-solid electrolyte membranes with percolated metal-organic frameworks for practical lithium-metal batteries. J. Energy Chem. 52, 354–360 (2020). https://doi.org/10.1016/j.jechem.2020.04.013
- Y.F. Xu, L.N. Gao, L. Shen, Q.Q. Liu, Y.Y. Zhu et al., Ion-transport-rectifying layer enables Li-metal batteries with high energy density. Matter 3(5), 1685–1700 (2020). https://doi.org/10.1016/j.matt.2020.08.011
- Q.X. Mu, H. Wang, X.Y. Gu, Z.R. Stephen, C. Yen et al., Biconcave carbon nanodisks for enhanced drug accumulation and chemo-photothermal tumor therapy. Adv. Healthc. Mater. 8(8), 1801505 (2019). https://doi.org/10.1002/adhm.201801505
- C.M. Yu, D.P. Qian, X. Huang, F.F. Han, N. Bao et al., Construction of biconcave hemoglobin-based microcapsules and electrochemical evaluation for its ability of oxygen carry. Sens. Actuators B: Chem. 256, 217–225 (2018). https://doi.org/10.1016/j.snb.2017.09.166
- C.-H. Wang, C.-H. Yang, J.-K. Chang, Suitability of ionic liquid electrolytes for room-temperature sodium-ion battery applications. Chem. Commun. 52(72), 10890 (2016). https://doi.org/10.1039/c6cc04625h
- A. Ponrouch, A.R. Goñi, P.M. Rosa, High capacity hard carbon anodes for sodium ion batteries in additive free electrolyte. Electrochem. Commun. 27, 85–88 (2013). https://doi.org/10.1016/j.elecom.2012.10.038
- M. Dan-Hardi, C. Serre, T. Frot, L. Rozes, G. Maurin et al., A new photoactive crystalline highly porous titanium(IV) dicarboxylate. J. Am. Chem. Soc. 131(31), 10857–10859 (2009). https://doi.org/10.1021/ja903726m
- J. Evans, C.A. Vincent, P.G. Bruce, Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28(13), 2324–2328 (1987). https://doi.org/10.1016/0032-3861(87)90394-6
- M. Shakourian-Fard, G. Kamath, K. Smith, H. Xiong, S.K.R.S. Sankaranarayanan, Trends in Na-ion solvation with alkyl-carbonate electrolytes for sodium-ion batteries: insights from first principles calculations. J. Phys. Chem. C 119(40), 22747–22759 (2015). https://doi.org/10.1021/acs.jpcc.5b04706
- Z. Chang, Y. Qiao, H. Deng, H.J. Yang, P. He et al., A liquid electrolyte with de-solvated lithium ions for lithium-metal battery. Joule 4(8), 1776–1789 (2020). https://doi.org/10.1016/j.joule.2020.06.011
- C.X. Liu, Y.Z. Ouyang, B. Jia, Z.Q. Zhu, J.B. Shi et al., Lead-enhanced gas-phase stability of multiply charged EDTA anions: a combined experimental and theoretical study. J. Mass Spectrom. 47(6), 769–777 (2012). https://doi.org/10.1002/jms.3014
- J. Barman, S. Acharya, C.Z. Zhou, S. Chatterjee, A. Engstrom et al., Non-identical electronic characters of the internucleotidic phosphates in RNA modulate the chemical reactivity of the phosphodiester bonds. Org. Biomol. Chem. 4(5), 928–941 (2006). https://doi.org/10.1039/b516733g
- A.V. Shchukarev, D.V. Korolkov, XPS study of group IA carbonates. Cent. Eur. J. Chem. 2(2), 347–362 (2004). https://doi.org/10.2478/BF02475578
- G. Beamson, D. Briggs, High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database, 1st edn. (Whliy, New York, 1992), p. 295
- C.Y.Y. Tang, Y.-N. Kwon, J.O. Leckie, Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes. Desalination 242(1), 149–167 (2009). https://doi.org/10.1016/j.desal.2008.04
- Z.Q. Wang, Z.J. Wang, L.Y. Yang, H.B. Wang, Y.L. Song et al., Boosting interfacial Li+ transport with a MOF-based ionic conductor for solid-state batteries. Nano Energy 49, 580–587 (2018). https://doi.org/10.1016/j.nanoen.2018.04.076
References
A. Sternberg, A. Bardow, Power-to-what? Environmental assessment of energy storage systems. Energy Environ. Sci. 8(2), 389–400 (2015). https://doi.org/10.1039/c4ee03051f
H.S. Chen, T.N. Cong, W. Yang, C.Q. Tan, Y.L. Li et al., Progress in electrical energy storage system: a critical review. Progr. Nat. Sci. 19(3), 291–312 (2009). https://doi.org/10.1016/j.pnsc.2008.07.014
M.D. Slater, D. Kim, E. Lee, C.S. Johnson, Sodium-ion batteries. Adv. Funct. Mater. 23(8), 947–958 (2013). https://doi.org/10.1002/adfm.201200691
H.S. Li, L.L. Peng, Y. Zhu, D.H. Chen, X.G. Zhang et al., An advanced high-energy sodium ion full battery based on nanostructured Na2Ti3O7/VOPO4 layered materials. Energy Environ. Sci. 9(11), 3399–3405 (2016). https://doi.org/10.1039/c6ee00794e
B. Zhang, G. Rousse, D. Foix, R. Dugas, D.A.D. Corte et al., Microsized Sn as advanced anodes in glyme-based electrolyte for Na-ion batteries. Adv. Mater. 28(44), 9824–9830 (2016). https://doi.org/10.1002/adma.201603212
X.Q. Ma, L. Zou, W.X. Zhao, Tailoring hollow microflower-shaped CoSe2 anodes in sodium ion batteries with high cycling stability. Chem. Commun. 54(74), 10507–10510 (2018). https://doi.org/10.1039/c8cc04426k
G.L. Woods, K.L. White, D.K. Vanderwall, G.P. Li, K.I. Aston et al., A mule cloned from fetal cells by nuclear transfer. Science 301(5636), 1063 (2003). https://doi.org/10.1126/science.1086743
H.-D. Nguyen, G.-T. Kim, J.L. Shi, E. Paillard, P. Judeinstein et al., Nanostructured multi-block copolymer single-ion conductors for safer high-performance lithium batteries. Energy Environ. Sci. 11(11), 3298–3309 (2018). https://doi.org/10.1039/c8ee02093k
Y.X. Huang, L.Z. Zhao, L. Li, M. Xie, F. Wu et al., Electrolytes and electrolyte/electrode interfaces in sodium-ion batteries: from scientific research to practical application. Adv. Mater. 31(21), 1808393 (2019). https://doi.org/10.1002/adma.201808393
Y. Lu, L. Li, Q. Zhang, Z.Q. Niu, J. Chen, Electrolyte and interface engineering for solid-state sodium batteries. Joule 2(9), 1747–1770 (2018). https://doi.org/10.1016/j.joule.2018.07.028
L. Xu, S. Tang, Y. Cheng, K.Y. Wang, J.Y. Liang et al., Interfaces in solid-state lithium batteries. Joule 2(10), 1991–2015 (2018). https://doi.org/10.1016/j.joule.2018.07.009
W.R. Hou, X.W. Guo, X.Y. Shen, K. Amine, H.J. Yu et al., Solid electrolytes and interfaces in all-solid-state sodium batteries: progress and perspective. Nano Energy 52, 279–291 (2018). https://doi.org/10.1016/j.nanoen.2018.07.036
L. Porcarelli, A.S. Shaplov, F. Bella, J.R. Nair, D. Mecerreyes et al., Single-ion conducting polymer electrolytes for lithium metal polymer batteries that operate at ambient temperature. ACS Energy Lett. 1(4), 678–682 (2016). https://doi.org/10.1021/acsenergylett.6b00216
H.C. Gao, L.G. Xue, S. Xin, K. Park, J.B. Goodenough, A plastic-crystal electrolyte interphase for all-solid-state sodium batteries. Angew. Chem. Int. Ed. 56(20), 5541–5545 (2017). https://doi.org/10.1002/anie.201702003
F. Lv, Z.Y. Wang, L.Y. Shi, J.F. Zhu, K. Edström et al., Challenges and development of composite solid-state electrolytes for high-performance lithium ion batteries. J. Power Sour. 441, 227175 (2019). https://doi.org/10.1016/j.jpowsour.2019.227175
H.L. Li, M. Eddaoudi, M. O’Keeffe, O.M. Yaghi, Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nat. Energy 402(6759), 276–279 (1999). https://doi.org/10.1038/46248
H.-C. Zhou, J.R. Long, O.M. Yaghi, Introduction to metal-organic frameworks. Chem. Rev. 112(2), 673–674 (2012). https://doi.org/10.1021/cr300014x
M. Li, D. Li, M. O’Keeffe, O.M. Yaghi, Topological analysis of metal-organic frameworks with polytopic linkers and/or multiple building units and the minimal transitivity principle. Chem. Rev. 114(2), 1343–1370 (2014). https://doi.org/10.1021/cr400392k
W.G. Lu, Z.W. Wei, Z.-Y. Gu, T.-F. Liu, J. Park et al., Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 43(16), 5561–5593 (2014). https://doi.org/10.1039/c4cs00003j
R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa et al., High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 319(5865), 939–943 (2008). https://doi.org/10.1126/science.1152516
Z.Q. Wang, R. Tan, H.B. Wang, L.Y. Yang, J.T. Hu et al., A metal-organic-framework-based electrolyte with nanowetted interfaces for high-energy-density solid-state lithium battery. Adv. Mater. 30(2), 1704436 (2018). https://doi.org/10.1002/adma.201704436
Z.Q. Wang, J.T. Hu, L. Han, Z.J. Wang, H.B. Wang et al., A MOF-based single-ion Zn2+ solid electrolyte leading to dendrite-free rechargeable Zn batteries. Nano Energy 56, 92–99 (2019). https://doi.org/10.1016/j.nanoen.2018.11.038
S. Ma, L. Shen, Q. Liu, W. Shi, C. Zhang et al., Class of solid-like electrolytes for rechargeable batteries based on metal-organic frameworks infiltrated with liquid electrolytes. ACS Appl. Mater. Interfaces 12(39), 43824–43832 (2020). https://doi.org/10.1021/acsami.0c13437
S.S. Park, Y. Tulchinsky, M. Dinca, Single-ion Li+, Na+, and Mg2+ solid electrolytes supported by a mesoporous anionic Cu-azolate metal-organic framework. J. Am. Chem. Soc. 139(38), 13260–13263 (2017). https://doi.org/10.1021/jacs.7b06197
L. Shen, H.B. Wu, F. Liu, J.L. Brosmer, G.R. Shen et al., Creating lithium-ion electrolytes with biomimetic ionic channels in metal-organic frameworks. Adv. Mater. 30(23), e1707476 (2018). https://doi.org/10.1002/adma.201707476
Jo.E.C.J. Li, Q.Q. Liu, L.N. Gao, Y.F. Xu, X.Q. Kong et al., Quasi-solid electrolyte membranes with percolated metal-organic frameworks for practical lithium-metal batteries. J. Energy Chem. 52, 354–360 (2020). https://doi.org/10.1016/j.jechem.2020.04.013
Y.F. Xu, L.N. Gao, L. Shen, Q.Q. Liu, Y.Y. Zhu et al., Ion-transport-rectifying layer enables Li-metal batteries with high energy density. Matter 3(5), 1685–1700 (2020). https://doi.org/10.1016/j.matt.2020.08.011
Q.X. Mu, H. Wang, X.Y. Gu, Z.R. Stephen, C. Yen et al., Biconcave carbon nanodisks for enhanced drug accumulation and chemo-photothermal tumor therapy. Adv. Healthc. Mater. 8(8), 1801505 (2019). https://doi.org/10.1002/adhm.201801505
C.M. Yu, D.P. Qian, X. Huang, F.F. Han, N. Bao et al., Construction of biconcave hemoglobin-based microcapsules and electrochemical evaluation for its ability of oxygen carry. Sens. Actuators B: Chem. 256, 217–225 (2018). https://doi.org/10.1016/j.snb.2017.09.166
C.-H. Wang, C.-H. Yang, J.-K. Chang, Suitability of ionic liquid electrolytes for room-temperature sodium-ion battery applications. Chem. Commun. 52(72), 10890 (2016). https://doi.org/10.1039/c6cc04625h
A. Ponrouch, A.R. Goñi, P.M. Rosa, High capacity hard carbon anodes for sodium ion batteries in additive free electrolyte. Electrochem. Commun. 27, 85–88 (2013). https://doi.org/10.1016/j.elecom.2012.10.038
M. Dan-Hardi, C. Serre, T. Frot, L. Rozes, G. Maurin et al., A new photoactive crystalline highly porous titanium(IV) dicarboxylate. J. Am. Chem. Soc. 131(31), 10857–10859 (2009). https://doi.org/10.1021/ja903726m
J. Evans, C.A. Vincent, P.G. Bruce, Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28(13), 2324–2328 (1987). https://doi.org/10.1016/0032-3861(87)90394-6
M. Shakourian-Fard, G. Kamath, K. Smith, H. Xiong, S.K.R.S. Sankaranarayanan, Trends in Na-ion solvation with alkyl-carbonate electrolytes for sodium-ion batteries: insights from first principles calculations. J. Phys. Chem. C 119(40), 22747–22759 (2015). https://doi.org/10.1021/acs.jpcc.5b04706
Z. Chang, Y. Qiao, H. Deng, H.J. Yang, P. He et al., A liquid electrolyte with de-solvated lithium ions for lithium-metal battery. Joule 4(8), 1776–1789 (2020). https://doi.org/10.1016/j.joule.2020.06.011
C.X. Liu, Y.Z. Ouyang, B. Jia, Z.Q. Zhu, J.B. Shi et al., Lead-enhanced gas-phase stability of multiply charged EDTA anions: a combined experimental and theoretical study. J. Mass Spectrom. 47(6), 769–777 (2012). https://doi.org/10.1002/jms.3014
J. Barman, S. Acharya, C.Z. Zhou, S. Chatterjee, A. Engstrom et al., Non-identical electronic characters of the internucleotidic phosphates in RNA modulate the chemical reactivity of the phosphodiester bonds. Org. Biomol. Chem. 4(5), 928–941 (2006). https://doi.org/10.1039/b516733g
A.V. Shchukarev, D.V. Korolkov, XPS study of group IA carbonates. Cent. Eur. J. Chem. 2(2), 347–362 (2004). https://doi.org/10.2478/BF02475578
G. Beamson, D. Briggs, High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database, 1st edn. (Whliy, New York, 1992), p. 295
C.Y.Y. Tang, Y.-N. Kwon, J.O. Leckie, Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes. Desalination 242(1), 149–167 (2009). https://doi.org/10.1016/j.desal.2008.04
Z.Q. Wang, Z.J. Wang, L.Y. Yang, H.B. Wang, Y.L. Song et al., Boosting interfacial Li+ transport with a MOF-based ionic conductor for solid-state batteries. Nano Energy 49, 580–587 (2018). https://doi.org/10.1016/j.nanoen.2018.04.076