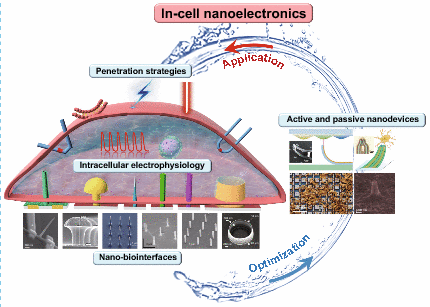
In-Cell Nanoelectronics: Opening the Door to Intracellular Electrophysiology
Corresponding Author: Ning Hu
Nano-Micro Letters,
Vol. 13 (2021), Article Number: 127
Abstract
Establishing a reliable electrophysiological recording platform is crucial for cardiology and neuroscience research. Noninvasive and label-free planar multitransistors and multielectrode arrays are conducive to perform the large-scale cellular electrical activity recordings, but the signal attenuation limits these extracellular devices to record subthreshold activities. In recent decade, in-cell nanoelectronics have been rapidly developed to open the door to intracellular electrophysiology. With the unique three-dimensional nanotopography and advanced penetration strategies, high-throughput and high-fidelity action potential like signal recordings is expected to be realized. This review summarizes in-cell nanoelectronics from versatile nano-biointerfaces, penetration strategies, active/passive nanodevices, systematically analyses the applications in electrogenic cells and especially evaluates the influence of nanodevices on the high-quality intracellular electrophysiological signals. Further, the opportunities, challenges and broad prospects of in-cell nanoelectronics are prospected, expecting to promote the development of in-cell electrophysiological platforms to meet the demand of theoretical investigation and clinical application.
Highlights:
1 The factors affecting in-cell nanoelectronics for electrophysiology recording were discussed from versatile nano-biointerfaces, penetration strategies, active and passive nanodevices.
2 The applications of in-cell nanodevices in cardiomyocyte and neuron were further reviewed and evaluated.
3 The current challenges of nanodevices for intracellular electrophysiology and their potential applications in biomedical fields were discussed.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- M. Levin, T. Thorlin, K.R. Robinson, T. Nogi, M. Mercola, Asymmetries in h+/k+-atpase and cell membrane potentials comprise a very early step in left-right patterning. Cell 111(1), 77–89 (2002). https://doi.org/10.1016/S0092-8674(02)00939-X
- D.J. Blackiston, K.A. McLaughlin, M. Levin, Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle 8(21), 3527–3536 (2009). https://doi.org/10.4161/cc.8.21.9888
- S. Sundelacruz, M. Levin, D.L. Kaplan, Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev. Rep. 5(3), 231–246 (2009). https://doi.org/10.1007/s12015-009-9080-2
- M. Jia, H. Dechiruji, J. Selberg, P. Pansodtee, J. Mathews et al., Bioelectronic control of chloride ions and concentration with Ag/AgCl contacts. APL Maters. 8(9), 091106 (2020). https://doi.org/10.1063/5.0013867
- V.P. Pai, J. Cervera, S. Mafe, V. Willocq, E.K. Lederer et al., HCN2 channel-induced rescue of brain teratogenesis via local and long-range bioelectric repair. Front. Cell Neurosci. 14, 136 (2020). https://doi.org/10.3389/fncel.2020.00136
- P.R. Rocha, P. Schlett, U. Kintzel, V. Mailänder, L.K. Vandamme et al., Electrochemical noise and impedance of au electrode/electrolyte interfaces enabling extracellular detection of glioma cell populations. Sci. Rep. 6(1), 1–10 (2016). https://doi.org/10.1038/srep34843
- T. Meyer, K.H. Boven, E. Gunther, M. Fejtl, Micro-electrode arrays in cardiac safety pharmacology - a novel tool to study qt interval prolongation. Drug Saf. 27(11), 763–772 (2004). https://doi.org/10.2165/00002018-200427110-00002
- J. Dunlop, M. Bowlby, R. Peri, D. Vasilyev, R. Arias, High-throughput electrophysiology: An emerging paradigm for ion-channel screening and physiology. Nat. Rev. Drug Discov. 7(4), 358–368 (2008). https://doi.org/10.1038/nrd2552
- R. Liu, R. Chen, A.T. Elthakeb, S.H. Lee, S. Hinckley et al., High density individually addressable nanowire arrays record intracellular activity from primary rodent and human stem cell derived neurons. Nano Lett. 17(5), 2757–2764 (2017). https://doi.org/10.1021/acs.nanolett.6b04752
- B.X.E. Desbiolles, E. de Coulon, A. Bertsch, S. Rohr, P. Renaud, Intracellular recording of cardiomyocyte action potentials with nanopatterned volcano-shaped microelectrode arrays. Nano Lett. 19(9), 6173–6181 (2019). https://doi.org/10.1021/acs.nanolett.9b02209
- A. Timmis, N. Townsend, C. Gale, R. Grobbee, N. Maniadakis et al., European society of cardiology: cardiovascular disease statistics 2017. Eur. Heart J. 39(7), 508–579 (2018). https://doi.org/10.1093/eurheartj/ehx628
- E.J. Benjamin, S.S. Virani, C.W. Callaway, A.M. Chamberlain, A.R. Chang et al., Heart disease and stroke statistics—2018 update: a report from the American heart association. Circulation 137, e67–e492 (2018). https://doi.org/10.1161/CIR.0000000000000558
- N.J. White, Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 7(8), 549–558 (2007). https://doi.org/10.1016/S1473-3099(07)70187-1
- P. Menna, E. Salvatorelli, G. Minotti, Cardiotoxicity of antitumor drugs. Chem. Res. Toxicol. 21(5), 978–989 (2008). https://doi.org/10.1021/tx800002r
- K.J. Schimmel, D.J. Richel, R.B. van den Brink, H.-J. Guchelaar, Cardiotoxicity of cytotoxic drugs. Cancer Treatment Rev. 30(2), 181–191 (2004). https://doi.org/10.1016/j.ctrv.2003.07.003
- R.S. Jiji, C.M. Kramer, M. Salerno, Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs. J. Nucl. Cardio. 19(2), 377–388 (2012). https://doi.org/10.1007/s12350-012-9512-2
- A.L. Hodgkin, A. Huxley, Action potentials recorded from inside a nerve fibre. Nature 144(3651), 710–711 (1939). https://doi.org/10.1038/144710a0
- B. Sakmann, E. Neher, Patch clamp techniques for studying ionic channels in excitable membranes. Annu. Rev. Physiol. 46(1), 455–472 (1984)
- D. Eytan, S. Marom, Dynamics and effective topology underlying synchronization in networks of cortical neurons. J. Neurosci. 26(33), 8465–8476 (2006). https://doi.org/10.1523/JNEUROSCI.1627-06.2006
- L. Berdondini, K. Imfeld, A. Maccione, M. Tedesco, S. Neukom, M. Koudelka-Hep, S. Martinoia, Active pixel sensor array for high spatio-temporal resolution electrophysiological recordings from single cell to large scale neuronal networks. Lab Chip 9(18), 2644–2651 (2009). https://doi.org/10.1039/b907394a
- D.S. Bassett, O. Sporns, Network neuroscience. Nat. Neurosci. 20(3), 353 (2017). https://doi.org/10.1038/nn.4502
- M. Hämäläinen, R. Hari, R.J. Ilmoniemi, J. Knuutila, O.V. Lounasmaa, Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys. 65(2), 413 (1993). https://doi.org/10.1103/RevModPhys.65.413
- S. Murakami, Y. Okada, Contributions of principal neocortical neurons to magnetoencephalography and electroencephalography signals. J. Phys. 575(3), 925–936 (2006). https://doi.org/10.1113/jphysiol.2006.105379
- N.K. Logothetis, What we can do and what we cannot do with fmri. Nature 453(7197), 869–878 (2008). https://doi.org/10.1038/nature06976
- M. Guye, G. Bettus, F. Bartolomei, P.J. Cozzone, Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. Magn. Reson. Mater. Phys. 23(5–6), 409–421 (2010). https://doi.org/10.1007/s10334-010-0205-z
- G. Buzsáki, C.A. Anastassiou, C. Koch, The origin of extracellular fields and currents—EEG, ECoG. LFP and spikes. Nat. Rev. Neurosci. 13(6), 407–420 (2012). https://doi.org/10.1038/nrn3241
- N.J. Holter, New method for heart studies: Continuous electrocardiography of active subjects over long periods is now practical. Science 134(3486), 1214–1220 (1961). https://doi.org/10.1126/science.134.3486.1214
- D.M. Mirvis, A.L. Goldberger, Electrocardiography. Heart Disease A Text Book of Cardiovascular Medicine, 2008.
- C. Thomas Jr., P. Springer, G. Loeb, Y. Berwald-Netter, L. Okun, A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp. Cell Res. 74(1), 61–66 (1972). https://doi.org/10.1016/0014-4827(72)90481-8
- G.W. Gross, E. Rieske, G. Kreutzberg, A. Meyer, A new fixed-array multi-microelectrode system designed for long-term monitoring of extracellular single unit neuronal activity in vitro. Neurosci. Lett. 6(2–3), 101–105 (1977). https://doi.org/10.1016/0304-3940(77)90003-9
- J. Pine, Recording action potentials from cultured neurons with extracellular microcircuit electrodes. J. Neurosci. Meth. 2(1), 19–31 (1980). https://doi.org/10.1016/0165-0270(80)90042-4
- P. Connolly, P. Clark, A.S.G. Curtis, J.A.T. Dow, C.D.W. Wilkinson, An extracellular microelectrode array for monitoring electrogenic cells in culture. Biosens. Bioelectron. 5(3), 223–234 (1990). https://doi.org/10.1016/0956-5663(90)80011-2
- G.W. Gross, B.K. Rhoades, D.L. Reust, F.U. Schwalm, Stimulation of monolayer networks in culture through thin-film indium-tin oxide recording electrodes. J. Neurosci. Meth. 50(2), 131–143 (1993). https://doi.org/10.1016/0165-0270(93)90001-8
- M. Reppel, F. Pillekamp, Z.J. Lu, M. Halbach, K. Brockmeier et al., Microelectrode arrays: A new tool to measure embryonic heart activity. J. Electrocardiol. 37, 104–109 (2004). https://doi.org/10.1016/j.jelectrocard.2004.08.033
- M. Cabello, H. Ge, C. Aracil, D. Moschou, P. Estrela et al., Extracellular electrophysiology in the prostate cancer cell model PC-3. Sensors 19(1), 139 (2019). https://doi.org/10.3390/s19010139
- M. Ribeiro, A. Elghajiji, S.P. Fraser, Z.D. Burke, D. Tosh et al., Human breast cancer cells demonstrate electrical excitability. Front. Neurosci. 14, 404 (2020). https://doi.org/10.3389/fnins.2020.00404
- J.C. Chang, G.J. Brewer, B.C. Wheeler, Microelectrode array recordings of patterned hippocampal neurons for four weeks. Biomed. Microdevices 2(4), 245–253 (2000). https://doi.org/10.1023/A:1009946920296
- S. Martinoia, L. Bonzano, M. Chiappalone, M. Tedesco, M. Marcoli, G. Maura, In vitro cortical neuronal networks as a new high-sensitive system for biosensing applications. Biosens. Bioelectron. 20(10), 2071–2078 (2005). https://doi.org/10.1016/j.bios.2004.09.012
- J. Erickson, A. Tooker, Y.-C. Tai, J. Pine, Caged neuron mea: A system for long-term investigation of cultured neural network connectivity. J. Neurosci. Meth. 175(1), 1–16 (2008). https://doi.org/10.1016/j.jneumeth.2008.07.02
- E.W. Keefer, A. Gramowski, D.A. Stenger, J.J. Pancrazio, G.W. Gross, Characterization of acute neurotoxic effects of trimethylolpropane phosphate via neuronal network biosensors. Biosens. Bioelectron. 16(7–8), 513–525 (2001). https://doi.org/10.1016/S0956-5663(01)00165-8
- J.V. Selinger, J.J. Pancrazio, G.W. Gross, Measuring synchronization in neuronal networks for biosensor applications. Biosens. Bioelectron. 19(7), 675–683 (2004). https://doi.org/10.1016/S0956-5663(03)00267-7
- Y. Nam, B.C. Wheeler, In vitro microelectrode array technology and neural recordings. Crit. Rev. Biomed. Eng. 39(1), 45–61 (2011). https://doi.org/10.1615/CritRevBiomedEng.v39.i1.40
- P. Fromherz, A. Offenhausser, T. Vetter, J. Weis, A neuron-silicon junction: a retzius cell of the leech on an insulated-gate field-effect transistor. Science 252(5010), 1290–1293 (1991). https://doi.org/10.1126/science.1925540
- M. Voelker, P. Fromherz, Signal transmission from individual mammalian nerve cell to field-effect transistor. Small 1(2), 206–210 (2005). https://doi.org/10.1002/smll.200400077
- F. Patolsky, B.P. Timko, G. Yu, Y. Fang, A.B. Greytak, G. Zheng et al., Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. Science 313(5790), 1100–1104 (2006). https://doi.org/10.1126/science.1128640
- T. Cohen-Karni, B.P. Timko, L.E. Weiss, C.M. Lieber, Flexible electrical recording from cells using nanowire transistor arrays. Proc. Natl. Acad. Sci. 106(18), 7309–7313 (2009). https://doi.org/10.1073/Proc.Natl.Acad.Sci.U.S.A.0902752106
- J.F. Eschermann, R. Stockmann, M. Hueske, X.T. Vu, S. Ingebrandt et al., Action potentials of hl-1 cells recorded with silicon nanowire transistors. Appl. Phys. Lett. 95(8), 083703 (2009). https://doi.org/10.1063/1.3194138
- A. Lambacher, M. Jenkner, M. Merz, B. Eversmann, R. Kaul et al., Electrical imaging of neuronal activity by multi-transistor-array (MTA) recording at 78 μm resolution. Appl. Phys. A 79(7), 1607–1611 (2004). https://doi.org/10.1007/s00339-004-2991-5
- T.J. Blanche, M.A. Spacek, J.F. Hetke, N.V. Swindale, Polytrodes: High-density silicon electrode arrays for large-scale multiunit recording. J. Neurophysiol. 93(5), 2987–3000 (2005). https://doi.org/10.1152/jn.01023.2004
- M. Hutzler, A. Lambacher, B. Eversmann, M. Jenkner, R. Thewes, P. Fromherz, High-resolution multitransistor array recording of electrical field potentials in cultured brain slices. J. Neurophysiol. 96(3), 1638–1645 (2006). https://doi.org/10.1152/jn.00347.2006
- U. Frey, U. Egert, F. Heer, S. Hafizovic, A. Hierlemann, Microelectronic system for high-resolution mapping of extracellular electric fields applied to brain slices. Biosens. Bioelectron. 24(7), 2191–2198 (2009). https://doi.org/10.1016/j.bios.2008.11.028
- T.S. Pui, A. Agarwal, F. Ye, N. Balasubramanian, P. Chen, CMOS-compatible nanowire sensor arrays for detection of cellular bioelectricity. Small 5(2), 208–212 (2009). https://doi.org/10.1002/smll.200800919
- R. Huys, D. Braeken, D. Jans, A. Stassen, N. Collaert et al., Single-cell recording and stimulation with a 16k micro-nail electrode array integrated on a 018 μm cmos chip. Lab Chip 12(7), 1274–1280 (2012). https://doi.org/10.1039/C2LC21037A
- T. Cohen-Karni, Q. Qing, Q. Li, Y. Fang, C.M. Lieber, Graphene and nanowire transistors for cellular interfaces and electrical recording. Nano Lett. 10(3), 1098–1102 (2010). https://doi.org/10.1021/nl1002608
- L.H. Hess, M. Jansen, V. Maybeck, M.V. Hauf, M. Seifert et al., Graphene transistor arrays for recording action potentials from electrogenic cells. Adv. Mater. 23(43), 5045–5049 (2011). https://doi.org/10.1002/adma.201102990
- Z. Cheng, J. Hou, Q. Zhou, T. Li, H. Li et al., Sensitivity limits and scaling of bioelectronic graphene transducers. Nano Lett. 13(6), 2902–2907 (2013). https://doi.org/10.1021/nl401276n
- M. Dankerl, S. Eick, B. Hofmann, M. Hauf, S. Ingebrandt et al., Diamond transistor array for extracellular recording from electrogenic cells. Adv. Funct. Mater. 19(18), 2915–2923 (2009). https://doi.org/10.1002/adfm.200900590
- E.W. Keefer, B.R. Botterman, M.I. Romero, A.F. Rossi, G.W. Gross, Carbon nanotube coating improves neuronal recordings. Nat. Nanotech. 3(7), 434–439 (2008). https://doi.org/10.1038/nnano.2008.174
- B. Sakmann, E. Neher, Patch clamp techniques for studying ionic channels in excitable membranes. Ann. Rev. Physiol. 46(1), 455–472 (1984). https://doi.org/10.1146/annurev.ph.46.030184.002323
- B. Hille, Ion Channels of Excitable Membranes. Edition 3 (2001)
- A. Molleman, Patch Clamping: An Introductory Guide to Patch Clamp Electrophysiology (2003)
- J.T. Davie, M.H. Kole, J.J. Letzkus, E.A. Rancz, N. Spruston et al., Dendritic patch-clamp recording. Nat. Protoc. 1(3), 1235–1247 (2006). https://doi.org/10.1038/nprot.2006.164
- B. Sakmann, Single-channel Recording (Springer, 2013)
- G. Wang, D.R. Wyskiel, W. Yang, Y. Wang, L.C. Milbern et al., An optogenetics- and imaging-assisted simultaneous multiple patch-clamp recording system for decoding complex neural circuits. Nat. Protoc. 10(3), 397–412 (2015). https://doi.org/10.1038/nprot.2015.019
- Y. Zhao, S. Inayat, D.A. Dikin, J.H. Singer, R.S. Ruoff et al., Patch clamp technique: Review of the current state of the art and potential contributions from nanoengineering. Proc. Inst. Mech. Eng. Part N: J. Nanoeng. Nanosys. 222(1), 1–11 (2009). https://doi.org/10.1243/17403499jnn149
- M. Bebarova, Advances in patch clamp technique: Towards higher quality and quantity. Gen. Physiol. Biophys. 31(2), 131–140 (2012). https://doi.org/10.4149/gpb_2012_016
- S. Cull-Candy, R. Miledi, I. Parker, Single glutamate-activated channels recorded from locust muscle fibres with perfused patch-clamp electrodes. J. Physiol. 321(1), 195–210 (1981). https://doi.org/10.1113/jphysiol.1981.sp013979
- G. Cota, C.M. Armstrong, Potassium channel" inactivation" induced by soft-glass patch pipettes. Biophys. J. 53(1), 107–109 (1988). https://doi.org/10.1016/S0006-3495(88)83071-6
- R.A. Levis, J.L. Rae, in [2] Constructing a Patch Clamp Setup. ed. by (Elsevier; 1992), pp. 14–66
- J.L. Rae, R.A. Levis, in [3] Glass Technology for Patch Clamp Electrodes. ed. by (Academic Press; 1992), pp. 66–92
- R.A. Levis, J.L. Rae, The use of quartz patch pipettes for low noise single channel recording. Biophys. J. 65(4), 1666–1677 (1993). https://doi.org/10.1016/S0006-3495(93)81224-4
- N. Fertig, R.H. Blick, J.C. Behrends, Whole cell patch clamp recording performed on a planar glass chip. Biophys. J. 82(6), 3056–3062 (2002). https://doi.org/10.1016/S0006-3495(02)75646-4
- A. Lepple-Wienhues, K. Ferlinz, A. Seeger, A. Schäfer, Flip the tip: An automated, high quality, cost-effective patch clamp screen. Receptor. Channel. 9(1), 13–17 (2003). https://doi.org/10.3109/10606820308257
- K. Schroeder, B. Neagle, D.J. Trezise, J. Worley, IonworksTM ht: A new high-throughput electrophysiology measurement platform. J. Biomol. Screen. 8(1), 50–64 (2003). https://doi.org/10.1177/1087057102239667
- J. Xu, A. Guia, D. Rothwarf, M. Huang, K. Sithiphong et al., A benchmark study with seal chipTM planar patch-clamp technology. Assay Drug Dev. Techn. 1(5), 675–684 (2003). https://doi.org/10.1089/154065803770381039
- A. Obergrussberger, C. Haarmann, S. Stölzle-Feix, N. Becker, A. Ohtsuki et al., in Automated Patch Clamp Recordings of Human Stem Cell-derived Cardiomyocytes. ed. by (Springer; 2017), pp. 57–82
- H. Cheng, W.J. Lederer, M.B. Cannell, Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science 262(5134), 740–744 (1993). https://doi.org/10.1126/science.8235594
- M.S. Siegel, E.Y. Isacoff, A genetically encoded optical probe of membrane voltage. Neuron 19(4), 735–741 (1997). https://doi.org/10.1016/S0896-6273(00)80955-1
- A. Grinvald, R. Hildesheim, Vsdi: A new era in functional imaging of cortical dynamics. Nat. Rev. Neurosci. 5(11), 874–885 (2004). https://doi.org/10.1038/nrn1536
- A. Matiukas, B.G. Mitrea, M. Qin, A.M. Pertsov, A.G. Shvedko et al., Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium. Heart Rhythm 4(11), 1441–1451 (2007). https://doi.org/10.1016/j.hrthm.2007.07.012
- M. Scanziani, M. Häusser, Electrophysiology in the age of light. Nature 461(7266), 930–939 (2009). https://doi.org/10.1038/nature08540
- M. Warren, K.W. Spitzer, B.W. Steadman, T.D. Rees, P. Venable et al., High-precision recording of the action potential in isolated cardiomyocytes using the near-infrared fluorescent dye di-4-ANBDQBS. Am. J. Physiol. Heart Circ. Physiol. 299(4), H1271–H1281 (2010). https://doi.org/10.1152/ajpheart.00248.2010
- T.J. Herron, P. Lee, J. Jalife, Optical imaging of voltage and calcium in cardiac cells & tissues. Circ. Res. 110(4), 609–623 (2012). https://doi.org/10.1161/circresaha.111.247494
- A. Lopez-Izquierdo, M. Warren, M. Riedel, S. Cho, S. Lai et al., A near-infrared fluorescent voltage-sensitive dye allows for moderate-throughput electrophysiological analyses of human induced pluripotent stem cell-derived cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 307(9), H1370–H1377 (2014). https://doi.org/10.1152/ajpheart.00344.2014
- B.P. Timko, T. Cohen-Karni, Q. Qing, B. Tian, C.M. Lieber, Design and implementation of functional nanoelectronic interfaces with biomolecules, cells, and tissue using nanowire device arrays. IEEE Trans. Nanotechnol. 9(3), 269–280 (2009). https://doi.org/10.1109/TNANO.2009.2031807
- C.M. Lieber, Semiconductor nanowires: A platform for nanoscience and nanotechnology. MRS Bull. 36(12), 1052–1063 (2011). https://doi.org/10.1557/mrs.2011.269
- P.B. Kruskal, Z. Jiang, T. Gao, C.M. Lieber, Beyond the patch clamp: Nanotechnologies for intracellular recording. Neuron 86(1), 21–24 (2015). https://doi.org/10.1016/j.neuron.2015.01.004
- B. Tian, T. Cohen-Karni, Q. Qing, X. Duan, P. Xie et al., Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science 329(5993), 830–834 (2010). https://doi.org/10.1126/science.1192033
- M.E. Spira, A. Hai, Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotech. 8(2), 83–94 (2013). https://doi.org/10.1038/nnano.2012.265
- N. Hu, D. Xu, J. Fang, H. Li, J. Mo, M. Zhou, B. Li, H. J. Chen, T. Zhang, J. Feng, T. Hang, W. Xia, X. Chen, X. Liu, G. He, X. Xie. Intracellular recording of cardiomyocyte action potentials by nanobranched microelectrode array. Biosens Bioelectron. 169(112588) (2020). https://doi.org/10.1016/j.bios.2020.112588
- J.T. Robinson, M. Jorgolli, A.K. Shalek, M.H. Yoon, R.S. Gertner et al., Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits. Nat. Nanotech. 7(3), 180–184 (2012). https://doi.org/10.1038/nnano.2011.249
- C. Xie, Z. Lin, L. Hanson, Y. Cui, B. Cui, Intracellular recording of action potentials by nanopillar electroporation. Nat. Nanotech. 7(3), 185–190 (2012). https://doi.org/10.1038/nnano.2012.8
- J. Abbott, T. Ye, L. Qin, M. Jorgolli, R.S. Gertner et al., Cmos nanoelectrode array for all-electrical intracellular electrophysiological imaging. Nat. Nanotech. 12(5), 460–466 (2017). https://doi.org/10.1038/nnano.2017.3
- J. Abbott, T. Ye, D. Ham, H. Park, Optimizing nanoelectrode arrays for scalable intracellular electrophysiology. Acc. Chem. Res. 51(3), 600–608 (2018). https://doi.org/10.1021/acs.accounts.7b00519
- J. Abbott, T. Ye, K. Krenek, R.S. Gertner, S. Ban et al., A nanoelectrode array for obtaining intracellular recordings from thousands of connected neurons. Nat. Biomed. Eng. 4(2), 232–241 (2020). https://doi.org/10.1038/s41551-019-0455-7
- Z.C. Lin, A.F. McGuire, P.W. Burridge, E. Matsa, H.Y. Lou et al., Accurate nanoelectrode recording of human pluripotent stem cell-derived cardiomyocytes for assaying drugs and modeling disease. Microsyst. Nanoeng. 3, 16080 (2017). https://doi.org/10.1038/micronano.2016.80
- M. Dipalo, G. Melle, L. Lovato, A. Jacassi, F. Santoro et al., Plasmonic meta-electrodes allow intracellular recordings at network level on high-density CMOS-multi-electrode arrays. Nat. Nanotech. 13(10), 965–971 (2018). https://doi.org/10.1038/s41565-018-0222-z
- A. Hai, J. Shappir, M.E. Spira, In-cell recordings by extracellular microelectrodes. Nat. Methods 7(3), 200–202 (2010). https://doi.org/10.1038/nmeth.1420
- A. Hai, J. Shappir, M.E. Spira, Long-term, multisite, parallel, in-cell recording and stimulation by an array of extracellular microelectrodes. J. Neurophysiol. 104(1), 559–568 (2010). https://doi.org/10.1152/jn.00265.2010
- A. Hai, M.E. Spira, On-chip electroporation, membrane repair dynamics and transient in-cell recordings by arrays of gold mushroom-shaped microelectrodes. Lab Chip 12(16), 2865–2873 (2012). https://doi.org/10.1039/c2lc40091j
- M. Dipalo, H. Amin, L. Lovato, F. Moia, V. Caprettini et al., Intracellular and extracellular recording of spontaneous action potentials in mammalian neurons and cardiac cells with 3d plasmonic nanoelectrodes. Nano Lett. 17(6), 3932–3939 (2017). https://doi.org/10.1021/acs.nanolett.7b01523
- Z.C. Lin, C. Xie, Y. Osakada, Y. Cui, B. Cui, Iridium oxide nanotube electrodes for sensitive and prolonged intracellular measurement of action potentials. Nat. Commun. 5, 3206 (2014). https://doi.org/10.1038/ncomms4206
- X. Duan, R. Gao, P. Xie, T. Cohen-Karni, Q. Qing et al., Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor. Nat. Nanotech. 7(3), 174–179 (2011). https://doi.org/10.1038/nnano.2011.223
- R. Gao, S. Strehle, B. Tian, T. Cohen-Karni, P. Xie et al., Outside looking in: Nanotube transistor intracellular sensors. . Nano Lett. 12(6), 3329–3333 (2012). https://doi.org/10.1021/nl301623p
- Z. Jiang, Q. Qing, P. Xie, R. Gao, C.M. Lieber, Kinked p-n junction nanowire probes for high spatial resolution sensing and intracellular recording. Nano Lett. 12(3), 1711–1716 (2012). https://doi.org/10.1021/nl300256r
- L. Xu, Z. Jiang, Q. Qing, L. Mai, Q. Zhang et al., Design and synthesis of diverse functional kinked nanowire structures for nanoelectronic bioprobes. Nano Lett. 13(2), 746–751 (2013). https://doi.org/10.1021/nl304435z
- T.M. Fu, X. Duan, Z. Jiang, X. Dai, P. Xie et al., Sub-10-nm intracellular bioelectronic probes from nanowire-nanotube heterostructures. Proc. Natl. Acad. Sci. USA 111(4), 1259–1264 (2014). https://doi.org/10.1073/Proc.Natl.Acad.Sci.U.S.A.1323389111
- Q. Qing, Z. Jiang, L. Xu, R. Gao, L. Mai et al., Free-standing kinked nanowire transistor probes for targeted intracellular recording in three dimensions. Nat. Nanotech. 9(2), 142–147 (2014). https://doi.org/10.1038/nnano.2013.273
- L. Xu, Z. Jiang, L. Mai, Q. Qing, Multiplexed free-standing nanowire transistor bioprobe for intracellular recording: A general fabrication strategy. Nano Lett. 14(6), 3602–3607 (2014). https://doi.org/10.1021/nl5012855
- Y. Zhao, S.S. You, A. Zhang, J.H. Lee, J. Huang et al., Scalable ultrasmall three-dimensional nanowire transistor probes for intracellular recording. Nat. Nanotech. 14(8), 783–790 (2019). https://doi.org/10.1038/s41565-019-0478-y
- M. Shein, A. Greenbaum, T. Gabay, R. Sorkin, M. David-Pur et al., Engineered neuronal circuits shaped and interfaced with carbon nanotube microelectrode arrays. Biomed. Microdevices 11(2), 495–501 (2009). https://doi.org/10.1007/s10544-008-9255-7
- J. Müller, M. Ballini, P. Livi, Y. Chen, M. Radivojevic et al., High-resolution cmos mea platform to study neurons at subcellular, cellular, and network levels. Lab Chip 15(13), 2767–2780 (2015). https://doi.org/10.1039/C5LC00133A
- A. Hai, A. Dormann, J. Shappir, S. Yitzchaik, C. Bartic et al., Spine-shaped gold protrusions improve the adherence and electrical coupling of neurons with the surface of micro-electronic devices. J. Royal Soc. Interface 6(41), 1153–1165 (2009). https://doi.org/10.1098/rsif.2009.0087
- K.-Y. Lee, I. Kim, S.-E. Kim, D.-W. Jeong, J.-J. Kim et al., Vertical nanowire probes for intracellular signaling of living cells. Nanoscale Res. Lett. 9(1), 56 (2014). https://doi.org/10.1186/1556-276X-9-56
- R. Elnathan, M. Kwiat, F. Patolsky, N.H. Voelcker, Engineering vertically aligned semiconductor nanowire arrays for applications in the life sciences. Nano Today 9(2), 172–196 (2014). https://doi.org/10.1016/j.nantod.2014.04.001
- A. Zhang, C.M. Lieber, Nano-bioelectronics. Chem. Rev. 116(1), 215–257 (2016). https://doi.org/10.1021/acs.chemrev.5b00608
- J.T. Hu, T.W. Odom, C.M. Lieber, Chemistry and physics in one dimension: Synthesis and properties of nanowires and nanotubes. Acc. Chem. Res. 32(5), 435–445 (1999). https://doi.org/10.1021/ar9700365
- S. Barth, F. Hernandez-Ramirez, J.D. Holmes, A. Romano-Rodriguez, Synthesis and applications of one-dimensional semiconductors. Prog. Mater. Sci. 55(6), 563–627 (2010). https://doi.org/10.1016/j.pmatsci.2010.02.001
- Q. Gao, V.G. Dubrovskii, P. Caroff, J. Wong-Leung, L. Li et al., Simultaneous selective-area and vapor-liquid-solid growth of InP nanowire arrays. Nano Lett. 16(7), 4361–4367 (2016). https://doi.org/10.1021/acs.nanolett.6b01461
- J. Westwater, D. Gosain, S. Tomiya, S. Usui, H. Ruda, Growth of silicon nanowires via gold/silane vapor–liquid–solid reaction. J. Vac. Sci. Technol. B 15(3), 554–557 (1997). https://doi.org/10.1116/1.589291
- R. Wagner, W. Ellis, Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 4(5), 89–90 (1964). https://doi.org/10.1007/BF00593955
- Y. Cui, L.J. Lauhon, M.S. Gudiksen, J.F. Wang, C.M. Lieber, Diameter-controlled synthesis of single-crystal silicon nanowires. Appl. Phys. Lett. 78(15), 2214–2216 (2001). https://doi.org/10.1063/1.1363692
- A.K. Shalek, J.T. Robinson, E.S. Karp, J.S. Lee, D.-R. Ahn et al., Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proc. Natl. Acad. Sci. USA 107(5), 1870–1875 (2010). https://doi.org/10.1073/Proc.Natl.Acad.Sci.U.S.A.0909350107
- Y.J. Hwang, C. Hahn, B. Liu, P. Yang, Photoelectrochemical properties of tio2 nanowire arrays: A study of the dependence on length and atomic layer deposition coating. ACS Nano 6(6), 5060–5069 (2012). https://doi.org/10.1021/nn300679d
- S.M. George, Atomic layer deposition: An overview. Chem. Rev. 110(1), 111–131 (2010). https://doi.org/10.1021/cr900056b
- H.J. Joyce, Q. Gao, H.H. Tan, C. Jagadish, Y. Kim et al., Iii-v semiconductor nanowires for optoelectronic device applications. Prog. Quant. Electron. 35(2–3), 23–75 (2011). https://doi.org/10.1016/j.pquantelec.2011.03.002
- K. Ogata, K. Maejima, S. Fujita, S. Fujita, Growth mode control of ZnO toward nanorod structures or high-quality layered structures by metal-organic vapor phase epitaxy. J. Cryst. Growth 248, 25–30 (2003). https://doi.org/10.1016/S0022-0248(02)01843-2
- D. Whang, S. Jin, C.M. Lieber, Nanolithography using hierarchically assembled nanowire masks. Nano Lett. 3(7), 951–954 (2003). https://doi.org/10.1021/nl034268a
- T. Mårtensson, P. Carlberg, M. Borgström, L. Montelius, W. Seifert et al., Nanowire arrays defined by nanoimprint lithography. Nano Lett. 4(4), 699–702 (2004). https://doi.org/10.1021/nl035100s
- R. Juhasz, N. Elfström, J. Linnros, Controlled fabrication of silicon nanowires by electron beam lithography and electrochemical size reduction. Nano Lett. 5(2), 275–280 (2005). https://doi.org/10.1021/nl0481573
- Z. Huang, H. Fang, J. Zhu, Fabrication of silicon nanowire arrays with controlled diameter, length, and density. Adv. Mater. 19(5), 744–748 (2007). https://doi.org/10.1002/adma.200600892
- A. del Campo, E. Arzt, Fabrication approaches for generating complex micro-and nanopatterns on polymeric surfaces. Chem. Rev. 108(3), 911–945 (2008). https://doi.org/10.1021/cr050018y
- Y.Q. Fu, A. Colli, A. Fasoli, J.K. Luo, A.J. Flewitt et al., Deep reactive ion etching as a tool for nanostructure fabrication. J. Vac. Sci. Technol. B 27(3), 1520–1526 (2009). https://doi.org/10.1116/1.3065991
- Z. Huang, N. Geyer, P. Werner, J. De Boor, U. Gösele, Metal-assisted chemical etching of silicon: A review: In memory of prof. Ulrich gösele. Adv. Mater. 23(2), 285–308 (2011). https://doi.org/10.1002/adma.201001784
- H. Wang, M. Sun, K. Ding, M.T. Hill, C.-Z. Ning, A top-down approach to fabrication of high quality vertical heterostructure nanowire arrays. Nano Lett. 11(4), 1646–1650 (2011). https://doi.org/10.1021/nl2001132
- O. Staufer, S. Weber, C.P. Bengtson, H. Bading, A. Rustom et al., Adhesion stabilized en masse intracellular electrical recordings from multicellular assemblies. Nano Lett. 19(5), 3244–3255 (2019). https://doi.org/10.1021/acs.nanolett.9b00784
- X. Xie, A.M. Xu, M.R. Angle, N. Tayebi, P. Verma et al., Mechanical model of vertical nanowire cell penetration. Nano Lett. 13(12), 6002–6008 (2013). https://doi.org/10.1021/nl403201a
- L. Hanson, Z.C. Lin, C. Xie, Y. Cui, B. Cui, Characterization of the cell–nanopillar interface by transmission electron microscopy. Nano Lett. 12(11), 5815–5820 (2012). https://doi.org/10.1021/nl303163y
- A.M. Xu, A. Aalipour, S. Leal-Ortiz, A.H. Mekhdjian, X. Xie et al., Quantification of nanowire penetration into living cells. Nat. Commun. 5(1), 1–8 (2014). https://doi.org/10.1038/ncomms4613
- M. Dipalo, A.F. McGuire, H.Y. Lou, V. Caprettini, G. Melle et al., Cells adhering to 3d vertical nanostructures: Cell membrane reshaping without stable internalization. Nano Lett. 18(9), 6100–6105 (2018). https://doi.org/10.1021/acs.nanolett.8b03163
- B.D. Almquist, N.A. Melosh, Fusion of biomimetic stealth probes into lipid bilayer cores. Proc. Natl. Acad. Sci. USA 107(13), 5815–5820 (2010). https://doi.org/10.1073/Proc.Natl.Acad.Sci.U.S.A.0909250107
- S.J. Luck, An Introduction to the Event-related Potential Technique (MIT Press; 2014)
- B. Tian, C.M. Lieber, Nanowired bioelectric interfaces. Chem. Rev. 119(15), 9136–9152 (2019). https://doi.org/10.1021/acs.chemrev.8b00795
- F. Pei, B. Tian, Nanoelectronics for minimally invasive cellular recordings. Adv. Funct. Mater. 1906210 (2019). https://doi.org/10.1002/adfm.201906210
- G.W. Gross, A.N. Williams, J.H. Lucas, Recording of spontaneous activity with photoetched microelectrode surfaces from mouse spinal neurons in culture. J. Neurosci. Meth. 5(1–2), 13–22 (1982). https://doi.org/10.1016/0165-0270(82)90046-2
- W.G. Regehr, J. Pine, C.S. Cohan, M.D. Mischke, D.W. Tank, Sealing cultured invertebrate neurons to embedded dish electrodes facilitates long-term stimulation and recording. J. Neurosci. Meth. 30(2), 91–106 (1989). https://doi.org/10.1016/0165-0270(89)90055-1
- A.F. Johnstone, G.W. Gross, D.G. Weiss, O.H.-U. Schroeder, A. Gramowski et al., Microelectrode arrays: A physiologically based neurotoxicity testing platform for the 21st century. Neurotoxicology 31(4), 331–350 (2010). https://doi.org/10.1016/j.neuro.2010.04.001
- L.R. Hochberg, M.D. Serruya, G.M. Friehs, J.A. Mukand, M. Saleh et al., Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442(7099), 164–171 (2006). https://doi.org/10.1038/nature04970
- A. Berényi, Z. Somogyvári, A.J. Nagy, L. Roux, J.D. Long et al., Large-scale, high-density (up to 512 channels) recording of local circuits in behaving animals. J. Neurophysiol. 111(5), 1132–1149 (2014). https://doi.org/10.1152/jn.00785.2013
- Y. Kubota, S. Yamagiwa, H. Sawahata, S. Idogawa, S. Tsuruhara et al., Long nanoneedle-electrode devices for extracellular and intracellular recording in vivo. Sens. Actuat. B Chem. 258, 1287–1294 (2018). https://doi.org/10.1016/j.snb.2017.11.152
- M.J. Nelson, P. Pouget, E.A. Nilsen, C.D. Patten, J.D. Schall, Review of signal distortion through metal microelectrode recording circuits and filters. J. Neurosci. Meth. 169(1), 141–157 (2008). https://doi.org/10.1016/j.jneumeth.2007.12.010
- P. Fromherz, Semiconductor chips with ion channels, nerve cells and brain. Physica E Low Dimens. Syst. Nanostruct. 16(1), 24–34 (2003). https://doi.org/10.1016/S1386-9477(02)00578-7
- A. Cerea, V. Caprettini, G. Bruno, L. Lovato, G. Melle et al., De Angelis: selective intracellular delivery and intracellular recordings combined in mea biosensors. Lab Chip 18(22), 3492–3500 (2018). https://doi.org/10.1039/c8lc00435h
- D.M. Bers, Cardiac excitation–contraction coupling. Nature 415(6868), 198–205 (2002). https://doi.org/10.1038/415198a
- D.P. Zipes, J. Jalife, Cardiac Electrophysiology: From Cell to Bedside E-book: Expert Consult (Elsevier Health Sciences, 2009)
- L.F. Santana, E.P. Cheng, W.J. Lederer, How does the shape of the cardiac action potential control calcium signaling and contraction in the heart? J. Mol. Cell Cardiol. 49(6), 901–903 (2010). https://doi.org/10.1016/j.yjmcc.2010.09.005
- J.M. Nerbonne, R.S. Kass, Molecular physiology of cardiac repolarization. Physiol. Rev. 85(4), 1205–1253 (2005). https://doi.org/10.1152/physrev.00002.2005
- P. Johnson, A. Freedberg, J. Marshall, Action of thyroid hormone on the transmembrane potentials from sinoatrial node cells and atrial muscle cells in isolated atria of rabbits. Cardiology 58(5), 273–289 (1973). https://doi.org/10.1159/000169643
- D. DiFrancesco, Pacemaker mechanisms in cardiac tissue. Ann. Rev. Physiol. 55(1), 455–472 (1993). https://doi.org/10.1146/annurev.ph.55.030193.002323
- E. Carmeliet, J. Vereecke, Adrenaline and the plateau phase of the cardiac action potential. Pflugers Arch. 313(4), 300–315 (1969). https://doi.org/10.1063/1.1753975
- C.-H. Luo, Y. Rudy, A model of the ventricular cardiac action potential: Depolarization, repolarization, and their interaction. Circ. Res. 68(6), 1501–1526 (1991). https://doi.org/10.1161/01.RES.68.6.1501
- Y. Rudy, Molecular basis of cardiac action potential repolarization. Ann. N. Y. Acad. Sci. 1123(1), 113–118 (2008). https://doi.org/10.1196/annals.1420.013
- N. Shmoel, N. Rabieh, S.M. Ojovan, H. Erez, E. Maydan et al., Multisite electrophysiological recordings by self-assembled loose-patch-like junctions between cultured hippocampal neurons and mushroom-shaped microelectrodes. Sci. Rep. 6, 27110 (2016). https://doi.org/10.1038/srep27110
- A.C. Dolphin, A. Lee, Presynaptic calcium channels: Specialized control of synaptic neurotransmitter release. Nat. Rev. Neurosci. 21(4), 213–229 (2020). https://doi.org/10.1038/s41583-020-0278-2
- E.D. Adrian, Wedensky inhibition in relation to theall-or-none’principle in nerve. J. Physiol. 46(4–5), 384–412 (1913). https://doi.org/10.1113/jphysiol.1913.sp001598
- E.D. Adrian, The Mechanism of Nervous Action, Electrical Studies of the Neurone (University of Pennsylvania Press; 2016)
- G. Stuart, J. Schiller, B. Sakmann, Action potential initiation and propagation in rat neocortical pyramidal neurons. J. Physiol. 505(3), 617–632 (1997). https://doi.org/10.1111/j.1469-7793.1997.617ba.x
- M. Dipalo, G.C. Messina, H. Amin, R. La Rocca, V. Shalabaeva et al., 3d plasmonic nanoantennas integrated with mea biosensors. Nanoscale 7(8), 3703–3711 (2015). https://doi.org/10.1039/c4nr05578k
- G. Bruno, N. Colistra, G. Melle, A. Cerea, A. Hubarevich et al., Microfluidic multielectrode arrays for spatially localized drug delivery and electrical recordings of primary neuronal cultures. Front. Bioeng. Biotechnol. 8, 626 (2020). https://doi.org/10.3389/fbioe.2020.00626
- A. Barbaglia, M. Dipalo, G. Melle, G. Iachetta, L. Deleye et al., Mirroring action potentials: Label-free, accurate, and noninvasive electrophysiological recordings of human-derived cardiomyocytes. Adv. Mater. 1, 2004234 (2021). https://doi.org/10.1002/adma.202004234
- C. Chiappini, J.O. Martinez, E. De Rosa, C.S. Almeida, E. Tasciotti et al., Biodegradable nanoneedles for localized delivery of nanops in vivo: exploring the biointerface. ACS Nano 9(5), 5500–5509 (2015). https://doi.org/10.1021/acsnano.5b01490
- Y. Cao, M. Hjort, H. Chen, F. Birey, S.A. Leal-Ortiz et al., Nondestructive nanostraw intracellular sampling for longitudinal cell monitoring. Proc. Natl. Acad. Sci. USA 114(10), E1866–E1874 (2017). https://doi.org/10.1073/pnas.1615375114
- A.M. Xu, D.S. Wang, P. Shieh, Y. Cao, N.A. Melosh, Direct intracellular delivery of cell-impermeable probes of protein glycosylation by using nanostraws. ChemBioChem 18(7), 623–628 (2017). https://doi.org/10.1002/cbic.201600689
References
M. Levin, T. Thorlin, K.R. Robinson, T. Nogi, M. Mercola, Asymmetries in h+/k+-atpase and cell membrane potentials comprise a very early step in left-right patterning. Cell 111(1), 77–89 (2002). https://doi.org/10.1016/S0092-8674(02)00939-X
D.J. Blackiston, K.A. McLaughlin, M. Levin, Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle 8(21), 3527–3536 (2009). https://doi.org/10.4161/cc.8.21.9888
S. Sundelacruz, M. Levin, D.L. Kaplan, Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev. Rep. 5(3), 231–246 (2009). https://doi.org/10.1007/s12015-009-9080-2
M. Jia, H. Dechiruji, J. Selberg, P. Pansodtee, J. Mathews et al., Bioelectronic control of chloride ions and concentration with Ag/AgCl contacts. APL Maters. 8(9), 091106 (2020). https://doi.org/10.1063/5.0013867
V.P. Pai, J. Cervera, S. Mafe, V. Willocq, E.K. Lederer et al., HCN2 channel-induced rescue of brain teratogenesis via local and long-range bioelectric repair. Front. Cell Neurosci. 14, 136 (2020). https://doi.org/10.3389/fncel.2020.00136
P.R. Rocha, P. Schlett, U. Kintzel, V. Mailänder, L.K. Vandamme et al., Electrochemical noise and impedance of au electrode/electrolyte interfaces enabling extracellular detection of glioma cell populations. Sci. Rep. 6(1), 1–10 (2016). https://doi.org/10.1038/srep34843
T. Meyer, K.H. Boven, E. Gunther, M. Fejtl, Micro-electrode arrays in cardiac safety pharmacology - a novel tool to study qt interval prolongation. Drug Saf. 27(11), 763–772 (2004). https://doi.org/10.2165/00002018-200427110-00002
J. Dunlop, M. Bowlby, R. Peri, D. Vasilyev, R. Arias, High-throughput electrophysiology: An emerging paradigm for ion-channel screening and physiology. Nat. Rev. Drug Discov. 7(4), 358–368 (2008). https://doi.org/10.1038/nrd2552
R. Liu, R. Chen, A.T. Elthakeb, S.H. Lee, S. Hinckley et al., High density individually addressable nanowire arrays record intracellular activity from primary rodent and human stem cell derived neurons. Nano Lett. 17(5), 2757–2764 (2017). https://doi.org/10.1021/acs.nanolett.6b04752
B.X.E. Desbiolles, E. de Coulon, A. Bertsch, S. Rohr, P. Renaud, Intracellular recording of cardiomyocyte action potentials with nanopatterned volcano-shaped microelectrode arrays. Nano Lett. 19(9), 6173–6181 (2019). https://doi.org/10.1021/acs.nanolett.9b02209
A. Timmis, N. Townsend, C. Gale, R. Grobbee, N. Maniadakis et al., European society of cardiology: cardiovascular disease statistics 2017. Eur. Heart J. 39(7), 508–579 (2018). https://doi.org/10.1093/eurheartj/ehx628
E.J. Benjamin, S.S. Virani, C.W. Callaway, A.M. Chamberlain, A.R. Chang et al., Heart disease and stroke statistics—2018 update: a report from the American heart association. Circulation 137, e67–e492 (2018). https://doi.org/10.1161/CIR.0000000000000558
N.J. White, Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 7(8), 549–558 (2007). https://doi.org/10.1016/S1473-3099(07)70187-1
P. Menna, E. Salvatorelli, G. Minotti, Cardiotoxicity of antitumor drugs. Chem. Res. Toxicol. 21(5), 978–989 (2008). https://doi.org/10.1021/tx800002r
K.J. Schimmel, D.J. Richel, R.B. van den Brink, H.-J. Guchelaar, Cardiotoxicity of cytotoxic drugs. Cancer Treatment Rev. 30(2), 181–191 (2004). https://doi.org/10.1016/j.ctrv.2003.07.003
R.S. Jiji, C.M. Kramer, M. Salerno, Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs. J. Nucl. Cardio. 19(2), 377–388 (2012). https://doi.org/10.1007/s12350-012-9512-2
A.L. Hodgkin, A. Huxley, Action potentials recorded from inside a nerve fibre. Nature 144(3651), 710–711 (1939). https://doi.org/10.1038/144710a0
B. Sakmann, E. Neher, Patch clamp techniques for studying ionic channels in excitable membranes. Annu. Rev. Physiol. 46(1), 455–472 (1984)
D. Eytan, S. Marom, Dynamics and effective topology underlying synchronization in networks of cortical neurons. J. Neurosci. 26(33), 8465–8476 (2006). https://doi.org/10.1523/JNEUROSCI.1627-06.2006
L. Berdondini, K. Imfeld, A. Maccione, M. Tedesco, S. Neukom, M. Koudelka-Hep, S. Martinoia, Active pixel sensor array for high spatio-temporal resolution electrophysiological recordings from single cell to large scale neuronal networks. Lab Chip 9(18), 2644–2651 (2009). https://doi.org/10.1039/b907394a
D.S. Bassett, O. Sporns, Network neuroscience. Nat. Neurosci. 20(3), 353 (2017). https://doi.org/10.1038/nn.4502
M. Hämäläinen, R. Hari, R.J. Ilmoniemi, J. Knuutila, O.V. Lounasmaa, Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys. 65(2), 413 (1993). https://doi.org/10.1103/RevModPhys.65.413
S. Murakami, Y. Okada, Contributions of principal neocortical neurons to magnetoencephalography and electroencephalography signals. J. Phys. 575(3), 925–936 (2006). https://doi.org/10.1113/jphysiol.2006.105379
N.K. Logothetis, What we can do and what we cannot do with fmri. Nature 453(7197), 869–878 (2008). https://doi.org/10.1038/nature06976
M. Guye, G. Bettus, F. Bartolomei, P.J. Cozzone, Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. Magn. Reson. Mater. Phys. 23(5–6), 409–421 (2010). https://doi.org/10.1007/s10334-010-0205-z
G. Buzsáki, C.A. Anastassiou, C. Koch, The origin of extracellular fields and currents—EEG, ECoG. LFP and spikes. Nat. Rev. Neurosci. 13(6), 407–420 (2012). https://doi.org/10.1038/nrn3241
N.J. Holter, New method for heart studies: Continuous electrocardiography of active subjects over long periods is now practical. Science 134(3486), 1214–1220 (1961). https://doi.org/10.1126/science.134.3486.1214
D.M. Mirvis, A.L. Goldberger, Electrocardiography. Heart Disease A Text Book of Cardiovascular Medicine, 2008.
C. Thomas Jr., P. Springer, G. Loeb, Y. Berwald-Netter, L. Okun, A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp. Cell Res. 74(1), 61–66 (1972). https://doi.org/10.1016/0014-4827(72)90481-8
G.W. Gross, E. Rieske, G. Kreutzberg, A. Meyer, A new fixed-array multi-microelectrode system designed for long-term monitoring of extracellular single unit neuronal activity in vitro. Neurosci. Lett. 6(2–3), 101–105 (1977). https://doi.org/10.1016/0304-3940(77)90003-9
J. Pine, Recording action potentials from cultured neurons with extracellular microcircuit electrodes. J. Neurosci. Meth. 2(1), 19–31 (1980). https://doi.org/10.1016/0165-0270(80)90042-4
P. Connolly, P. Clark, A.S.G. Curtis, J.A.T. Dow, C.D.W. Wilkinson, An extracellular microelectrode array for monitoring electrogenic cells in culture. Biosens. Bioelectron. 5(3), 223–234 (1990). https://doi.org/10.1016/0956-5663(90)80011-2
G.W. Gross, B.K. Rhoades, D.L. Reust, F.U. Schwalm, Stimulation of monolayer networks in culture through thin-film indium-tin oxide recording electrodes. J. Neurosci. Meth. 50(2), 131–143 (1993). https://doi.org/10.1016/0165-0270(93)90001-8
M. Reppel, F. Pillekamp, Z.J. Lu, M. Halbach, K. Brockmeier et al., Microelectrode arrays: A new tool to measure embryonic heart activity. J. Electrocardiol. 37, 104–109 (2004). https://doi.org/10.1016/j.jelectrocard.2004.08.033
M. Cabello, H. Ge, C. Aracil, D. Moschou, P. Estrela et al., Extracellular electrophysiology in the prostate cancer cell model PC-3. Sensors 19(1), 139 (2019). https://doi.org/10.3390/s19010139
M. Ribeiro, A. Elghajiji, S.P. Fraser, Z.D. Burke, D. Tosh et al., Human breast cancer cells demonstrate electrical excitability. Front. Neurosci. 14, 404 (2020). https://doi.org/10.3389/fnins.2020.00404
J.C. Chang, G.J. Brewer, B.C. Wheeler, Microelectrode array recordings of patterned hippocampal neurons for four weeks. Biomed. Microdevices 2(4), 245–253 (2000). https://doi.org/10.1023/A:1009946920296
S. Martinoia, L. Bonzano, M. Chiappalone, M. Tedesco, M. Marcoli, G. Maura, In vitro cortical neuronal networks as a new high-sensitive system for biosensing applications. Biosens. Bioelectron. 20(10), 2071–2078 (2005). https://doi.org/10.1016/j.bios.2004.09.012
J. Erickson, A. Tooker, Y.-C. Tai, J. Pine, Caged neuron mea: A system for long-term investigation of cultured neural network connectivity. J. Neurosci. Meth. 175(1), 1–16 (2008). https://doi.org/10.1016/j.jneumeth.2008.07.02
E.W. Keefer, A. Gramowski, D.A. Stenger, J.J. Pancrazio, G.W. Gross, Characterization of acute neurotoxic effects of trimethylolpropane phosphate via neuronal network biosensors. Biosens. Bioelectron. 16(7–8), 513–525 (2001). https://doi.org/10.1016/S0956-5663(01)00165-8
J.V. Selinger, J.J. Pancrazio, G.W. Gross, Measuring synchronization in neuronal networks for biosensor applications. Biosens. Bioelectron. 19(7), 675–683 (2004). https://doi.org/10.1016/S0956-5663(03)00267-7
Y. Nam, B.C. Wheeler, In vitro microelectrode array technology and neural recordings. Crit. Rev. Biomed. Eng. 39(1), 45–61 (2011). https://doi.org/10.1615/CritRevBiomedEng.v39.i1.40
P. Fromherz, A. Offenhausser, T. Vetter, J. Weis, A neuron-silicon junction: a retzius cell of the leech on an insulated-gate field-effect transistor. Science 252(5010), 1290–1293 (1991). https://doi.org/10.1126/science.1925540
M. Voelker, P. Fromherz, Signal transmission from individual mammalian nerve cell to field-effect transistor. Small 1(2), 206–210 (2005). https://doi.org/10.1002/smll.200400077
F. Patolsky, B.P. Timko, G. Yu, Y. Fang, A.B. Greytak, G. Zheng et al., Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. Science 313(5790), 1100–1104 (2006). https://doi.org/10.1126/science.1128640
T. Cohen-Karni, B.P. Timko, L.E. Weiss, C.M. Lieber, Flexible electrical recording from cells using nanowire transistor arrays. Proc. Natl. Acad. Sci. 106(18), 7309–7313 (2009). https://doi.org/10.1073/Proc.Natl.Acad.Sci.U.S.A.0902752106
J.F. Eschermann, R. Stockmann, M. Hueske, X.T. Vu, S. Ingebrandt et al., Action potentials of hl-1 cells recorded with silicon nanowire transistors. Appl. Phys. Lett. 95(8), 083703 (2009). https://doi.org/10.1063/1.3194138
A. Lambacher, M. Jenkner, M. Merz, B. Eversmann, R. Kaul et al., Electrical imaging of neuronal activity by multi-transistor-array (MTA) recording at 78 μm resolution. Appl. Phys. A 79(7), 1607–1611 (2004). https://doi.org/10.1007/s00339-004-2991-5
T.J. Blanche, M.A. Spacek, J.F. Hetke, N.V. Swindale, Polytrodes: High-density silicon electrode arrays for large-scale multiunit recording. J. Neurophysiol. 93(5), 2987–3000 (2005). https://doi.org/10.1152/jn.01023.2004
M. Hutzler, A. Lambacher, B. Eversmann, M. Jenkner, R. Thewes, P. Fromherz, High-resolution multitransistor array recording of electrical field potentials in cultured brain slices. J. Neurophysiol. 96(3), 1638–1645 (2006). https://doi.org/10.1152/jn.00347.2006
U. Frey, U. Egert, F. Heer, S. Hafizovic, A. Hierlemann, Microelectronic system for high-resolution mapping of extracellular electric fields applied to brain slices. Biosens. Bioelectron. 24(7), 2191–2198 (2009). https://doi.org/10.1016/j.bios.2008.11.028
T.S. Pui, A. Agarwal, F. Ye, N. Balasubramanian, P. Chen, CMOS-compatible nanowire sensor arrays for detection of cellular bioelectricity. Small 5(2), 208–212 (2009). https://doi.org/10.1002/smll.200800919
R. Huys, D. Braeken, D. Jans, A. Stassen, N. Collaert et al., Single-cell recording and stimulation with a 16k micro-nail electrode array integrated on a 018 μm cmos chip. Lab Chip 12(7), 1274–1280 (2012). https://doi.org/10.1039/C2LC21037A
T. Cohen-Karni, Q. Qing, Q. Li, Y. Fang, C.M. Lieber, Graphene and nanowire transistors for cellular interfaces and electrical recording. Nano Lett. 10(3), 1098–1102 (2010). https://doi.org/10.1021/nl1002608
L.H. Hess, M. Jansen, V. Maybeck, M.V. Hauf, M. Seifert et al., Graphene transistor arrays for recording action potentials from electrogenic cells. Adv. Mater. 23(43), 5045–5049 (2011). https://doi.org/10.1002/adma.201102990
Z. Cheng, J. Hou, Q. Zhou, T. Li, H. Li et al., Sensitivity limits and scaling of bioelectronic graphene transducers. Nano Lett. 13(6), 2902–2907 (2013). https://doi.org/10.1021/nl401276n
M. Dankerl, S. Eick, B. Hofmann, M. Hauf, S. Ingebrandt et al., Diamond transistor array for extracellular recording from electrogenic cells. Adv. Funct. Mater. 19(18), 2915–2923 (2009). https://doi.org/10.1002/adfm.200900590
E.W. Keefer, B.R. Botterman, M.I. Romero, A.F. Rossi, G.W. Gross, Carbon nanotube coating improves neuronal recordings. Nat. Nanotech. 3(7), 434–439 (2008). https://doi.org/10.1038/nnano.2008.174
B. Sakmann, E. Neher, Patch clamp techniques for studying ionic channels in excitable membranes. Ann. Rev. Physiol. 46(1), 455–472 (1984). https://doi.org/10.1146/annurev.ph.46.030184.002323
B. Hille, Ion Channels of Excitable Membranes. Edition 3 (2001)
A. Molleman, Patch Clamping: An Introductory Guide to Patch Clamp Electrophysiology (2003)
J.T. Davie, M.H. Kole, J.J. Letzkus, E.A. Rancz, N. Spruston et al., Dendritic patch-clamp recording. Nat. Protoc. 1(3), 1235–1247 (2006). https://doi.org/10.1038/nprot.2006.164
B. Sakmann, Single-channel Recording (Springer, 2013)
G. Wang, D.R. Wyskiel, W. Yang, Y. Wang, L.C. Milbern et al., An optogenetics- and imaging-assisted simultaneous multiple patch-clamp recording system for decoding complex neural circuits. Nat. Protoc. 10(3), 397–412 (2015). https://doi.org/10.1038/nprot.2015.019
Y. Zhao, S. Inayat, D.A. Dikin, J.H. Singer, R.S. Ruoff et al., Patch clamp technique: Review of the current state of the art and potential contributions from nanoengineering. Proc. Inst. Mech. Eng. Part N: J. Nanoeng. Nanosys. 222(1), 1–11 (2009). https://doi.org/10.1243/17403499jnn149
M. Bebarova, Advances in patch clamp technique: Towards higher quality and quantity. Gen. Physiol. Biophys. 31(2), 131–140 (2012). https://doi.org/10.4149/gpb_2012_016
S. Cull-Candy, R. Miledi, I. Parker, Single glutamate-activated channels recorded from locust muscle fibres with perfused patch-clamp electrodes. J. Physiol. 321(1), 195–210 (1981). https://doi.org/10.1113/jphysiol.1981.sp013979
G. Cota, C.M. Armstrong, Potassium channel" inactivation" induced by soft-glass patch pipettes. Biophys. J. 53(1), 107–109 (1988). https://doi.org/10.1016/S0006-3495(88)83071-6
R.A. Levis, J.L. Rae, in [2] Constructing a Patch Clamp Setup. ed. by (Elsevier; 1992), pp. 14–66
J.L. Rae, R.A. Levis, in [3] Glass Technology for Patch Clamp Electrodes. ed. by (Academic Press; 1992), pp. 66–92
R.A. Levis, J.L. Rae, The use of quartz patch pipettes for low noise single channel recording. Biophys. J. 65(4), 1666–1677 (1993). https://doi.org/10.1016/S0006-3495(93)81224-4
N. Fertig, R.H. Blick, J.C. Behrends, Whole cell patch clamp recording performed on a planar glass chip. Biophys. J. 82(6), 3056–3062 (2002). https://doi.org/10.1016/S0006-3495(02)75646-4
A. Lepple-Wienhues, K. Ferlinz, A. Seeger, A. Schäfer, Flip the tip: An automated, high quality, cost-effective patch clamp screen. Receptor. Channel. 9(1), 13–17 (2003). https://doi.org/10.3109/10606820308257
K. Schroeder, B. Neagle, D.J. Trezise, J. Worley, IonworksTM ht: A new high-throughput electrophysiology measurement platform. J. Biomol. Screen. 8(1), 50–64 (2003). https://doi.org/10.1177/1087057102239667
J. Xu, A. Guia, D. Rothwarf, M. Huang, K. Sithiphong et al., A benchmark study with seal chipTM planar patch-clamp technology. Assay Drug Dev. Techn. 1(5), 675–684 (2003). https://doi.org/10.1089/154065803770381039
A. Obergrussberger, C. Haarmann, S. Stölzle-Feix, N. Becker, A. Ohtsuki et al., in Automated Patch Clamp Recordings of Human Stem Cell-derived Cardiomyocytes. ed. by (Springer; 2017), pp. 57–82
H. Cheng, W.J. Lederer, M.B. Cannell, Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science 262(5134), 740–744 (1993). https://doi.org/10.1126/science.8235594
M.S. Siegel, E.Y. Isacoff, A genetically encoded optical probe of membrane voltage. Neuron 19(4), 735–741 (1997). https://doi.org/10.1016/S0896-6273(00)80955-1
A. Grinvald, R. Hildesheim, Vsdi: A new era in functional imaging of cortical dynamics. Nat. Rev. Neurosci. 5(11), 874–885 (2004). https://doi.org/10.1038/nrn1536
A. Matiukas, B.G. Mitrea, M. Qin, A.M. Pertsov, A.G. Shvedko et al., Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium. Heart Rhythm 4(11), 1441–1451 (2007). https://doi.org/10.1016/j.hrthm.2007.07.012
M. Scanziani, M. Häusser, Electrophysiology in the age of light. Nature 461(7266), 930–939 (2009). https://doi.org/10.1038/nature08540
M. Warren, K.W. Spitzer, B.W. Steadman, T.D. Rees, P. Venable et al., High-precision recording of the action potential in isolated cardiomyocytes using the near-infrared fluorescent dye di-4-ANBDQBS. Am. J. Physiol. Heart Circ. Physiol. 299(4), H1271–H1281 (2010). https://doi.org/10.1152/ajpheart.00248.2010
T.J. Herron, P. Lee, J. Jalife, Optical imaging of voltage and calcium in cardiac cells & tissues. Circ. Res. 110(4), 609–623 (2012). https://doi.org/10.1161/circresaha.111.247494
A. Lopez-Izquierdo, M. Warren, M. Riedel, S. Cho, S. Lai et al., A near-infrared fluorescent voltage-sensitive dye allows for moderate-throughput electrophysiological analyses of human induced pluripotent stem cell-derived cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 307(9), H1370–H1377 (2014). https://doi.org/10.1152/ajpheart.00344.2014
B.P. Timko, T. Cohen-Karni, Q. Qing, B. Tian, C.M. Lieber, Design and implementation of functional nanoelectronic interfaces with biomolecules, cells, and tissue using nanowire device arrays. IEEE Trans. Nanotechnol. 9(3), 269–280 (2009). https://doi.org/10.1109/TNANO.2009.2031807
C.M. Lieber, Semiconductor nanowires: A platform for nanoscience and nanotechnology. MRS Bull. 36(12), 1052–1063 (2011). https://doi.org/10.1557/mrs.2011.269
P.B. Kruskal, Z. Jiang, T. Gao, C.M. Lieber, Beyond the patch clamp: Nanotechnologies for intracellular recording. Neuron 86(1), 21–24 (2015). https://doi.org/10.1016/j.neuron.2015.01.004
B. Tian, T. Cohen-Karni, Q. Qing, X. Duan, P. Xie et al., Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science 329(5993), 830–834 (2010). https://doi.org/10.1126/science.1192033
M.E. Spira, A. Hai, Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotech. 8(2), 83–94 (2013). https://doi.org/10.1038/nnano.2012.265
N. Hu, D. Xu, J. Fang, H. Li, J. Mo, M. Zhou, B. Li, H. J. Chen, T. Zhang, J. Feng, T. Hang, W. Xia, X. Chen, X. Liu, G. He, X. Xie. Intracellular recording of cardiomyocyte action potentials by nanobranched microelectrode array. Biosens Bioelectron. 169(112588) (2020). https://doi.org/10.1016/j.bios.2020.112588
J.T. Robinson, M. Jorgolli, A.K. Shalek, M.H. Yoon, R.S. Gertner et al., Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits. Nat. Nanotech. 7(3), 180–184 (2012). https://doi.org/10.1038/nnano.2011.249
C. Xie, Z. Lin, L. Hanson, Y. Cui, B. Cui, Intracellular recording of action potentials by nanopillar electroporation. Nat. Nanotech. 7(3), 185–190 (2012). https://doi.org/10.1038/nnano.2012.8
J. Abbott, T. Ye, L. Qin, M. Jorgolli, R.S. Gertner et al., Cmos nanoelectrode array for all-electrical intracellular electrophysiological imaging. Nat. Nanotech. 12(5), 460–466 (2017). https://doi.org/10.1038/nnano.2017.3
J. Abbott, T. Ye, D. Ham, H. Park, Optimizing nanoelectrode arrays for scalable intracellular electrophysiology. Acc. Chem. Res. 51(3), 600–608 (2018). https://doi.org/10.1021/acs.accounts.7b00519
J. Abbott, T. Ye, K. Krenek, R.S. Gertner, S. Ban et al., A nanoelectrode array for obtaining intracellular recordings from thousands of connected neurons. Nat. Biomed. Eng. 4(2), 232–241 (2020). https://doi.org/10.1038/s41551-019-0455-7
Z.C. Lin, A.F. McGuire, P.W. Burridge, E. Matsa, H.Y. Lou et al., Accurate nanoelectrode recording of human pluripotent stem cell-derived cardiomyocytes for assaying drugs and modeling disease. Microsyst. Nanoeng. 3, 16080 (2017). https://doi.org/10.1038/micronano.2016.80
M. Dipalo, G. Melle, L. Lovato, A. Jacassi, F. Santoro et al., Plasmonic meta-electrodes allow intracellular recordings at network level on high-density CMOS-multi-electrode arrays. Nat. Nanotech. 13(10), 965–971 (2018). https://doi.org/10.1038/s41565-018-0222-z
A. Hai, J. Shappir, M.E. Spira, In-cell recordings by extracellular microelectrodes. Nat. Methods 7(3), 200–202 (2010). https://doi.org/10.1038/nmeth.1420
A. Hai, J. Shappir, M.E. Spira, Long-term, multisite, parallel, in-cell recording and stimulation by an array of extracellular microelectrodes. J. Neurophysiol. 104(1), 559–568 (2010). https://doi.org/10.1152/jn.00265.2010
A. Hai, M.E. Spira, On-chip electroporation, membrane repair dynamics and transient in-cell recordings by arrays of gold mushroom-shaped microelectrodes. Lab Chip 12(16), 2865–2873 (2012). https://doi.org/10.1039/c2lc40091j
M. Dipalo, H. Amin, L. Lovato, F. Moia, V. Caprettini et al., Intracellular and extracellular recording of spontaneous action potentials in mammalian neurons and cardiac cells with 3d plasmonic nanoelectrodes. Nano Lett. 17(6), 3932–3939 (2017). https://doi.org/10.1021/acs.nanolett.7b01523
Z.C. Lin, C. Xie, Y. Osakada, Y. Cui, B. Cui, Iridium oxide nanotube electrodes for sensitive and prolonged intracellular measurement of action potentials. Nat. Commun. 5, 3206 (2014). https://doi.org/10.1038/ncomms4206
X. Duan, R. Gao, P. Xie, T. Cohen-Karni, Q. Qing et al., Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor. Nat. Nanotech. 7(3), 174–179 (2011). https://doi.org/10.1038/nnano.2011.223
R. Gao, S. Strehle, B. Tian, T. Cohen-Karni, P. Xie et al., Outside looking in: Nanotube transistor intracellular sensors. . Nano Lett. 12(6), 3329–3333 (2012). https://doi.org/10.1021/nl301623p
Z. Jiang, Q. Qing, P. Xie, R. Gao, C.M. Lieber, Kinked p-n junction nanowire probes for high spatial resolution sensing and intracellular recording. Nano Lett. 12(3), 1711–1716 (2012). https://doi.org/10.1021/nl300256r
L. Xu, Z. Jiang, Q. Qing, L. Mai, Q. Zhang et al., Design and synthesis of diverse functional kinked nanowire structures for nanoelectronic bioprobes. Nano Lett. 13(2), 746–751 (2013). https://doi.org/10.1021/nl304435z
T.M. Fu, X. Duan, Z. Jiang, X. Dai, P. Xie et al., Sub-10-nm intracellular bioelectronic probes from nanowire-nanotube heterostructures. Proc. Natl. Acad. Sci. USA 111(4), 1259–1264 (2014). https://doi.org/10.1073/Proc.Natl.Acad.Sci.U.S.A.1323389111
Q. Qing, Z. Jiang, L. Xu, R. Gao, L. Mai et al., Free-standing kinked nanowire transistor probes for targeted intracellular recording in three dimensions. Nat. Nanotech. 9(2), 142–147 (2014). https://doi.org/10.1038/nnano.2013.273
L. Xu, Z. Jiang, L. Mai, Q. Qing, Multiplexed free-standing nanowire transistor bioprobe for intracellular recording: A general fabrication strategy. Nano Lett. 14(6), 3602–3607 (2014). https://doi.org/10.1021/nl5012855
Y. Zhao, S.S. You, A. Zhang, J.H. Lee, J. Huang et al., Scalable ultrasmall three-dimensional nanowire transistor probes for intracellular recording. Nat. Nanotech. 14(8), 783–790 (2019). https://doi.org/10.1038/s41565-019-0478-y
M. Shein, A. Greenbaum, T. Gabay, R. Sorkin, M. David-Pur et al., Engineered neuronal circuits shaped and interfaced with carbon nanotube microelectrode arrays. Biomed. Microdevices 11(2), 495–501 (2009). https://doi.org/10.1007/s10544-008-9255-7
J. Müller, M. Ballini, P. Livi, Y. Chen, M. Radivojevic et al., High-resolution cmos mea platform to study neurons at subcellular, cellular, and network levels. Lab Chip 15(13), 2767–2780 (2015). https://doi.org/10.1039/C5LC00133A
A. Hai, A. Dormann, J. Shappir, S. Yitzchaik, C. Bartic et al., Spine-shaped gold protrusions improve the adherence and electrical coupling of neurons with the surface of micro-electronic devices. J. Royal Soc. Interface 6(41), 1153–1165 (2009). https://doi.org/10.1098/rsif.2009.0087
K.-Y. Lee, I. Kim, S.-E. Kim, D.-W. Jeong, J.-J. Kim et al., Vertical nanowire probes for intracellular signaling of living cells. Nanoscale Res. Lett. 9(1), 56 (2014). https://doi.org/10.1186/1556-276X-9-56
R. Elnathan, M. Kwiat, F. Patolsky, N.H. Voelcker, Engineering vertically aligned semiconductor nanowire arrays for applications in the life sciences. Nano Today 9(2), 172–196 (2014). https://doi.org/10.1016/j.nantod.2014.04.001
A. Zhang, C.M. Lieber, Nano-bioelectronics. Chem. Rev. 116(1), 215–257 (2016). https://doi.org/10.1021/acs.chemrev.5b00608
J.T. Hu, T.W. Odom, C.M. Lieber, Chemistry and physics in one dimension: Synthesis and properties of nanowires and nanotubes. Acc. Chem. Res. 32(5), 435–445 (1999). https://doi.org/10.1021/ar9700365
S. Barth, F. Hernandez-Ramirez, J.D. Holmes, A. Romano-Rodriguez, Synthesis and applications of one-dimensional semiconductors. Prog. Mater. Sci. 55(6), 563–627 (2010). https://doi.org/10.1016/j.pmatsci.2010.02.001
Q. Gao, V.G. Dubrovskii, P. Caroff, J. Wong-Leung, L. Li et al., Simultaneous selective-area and vapor-liquid-solid growth of InP nanowire arrays. Nano Lett. 16(7), 4361–4367 (2016). https://doi.org/10.1021/acs.nanolett.6b01461
J. Westwater, D. Gosain, S. Tomiya, S. Usui, H. Ruda, Growth of silicon nanowires via gold/silane vapor–liquid–solid reaction. J. Vac. Sci. Technol. B 15(3), 554–557 (1997). https://doi.org/10.1116/1.589291
R. Wagner, W. Ellis, Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 4(5), 89–90 (1964). https://doi.org/10.1007/BF00593955
Y. Cui, L.J. Lauhon, M.S. Gudiksen, J.F. Wang, C.M. Lieber, Diameter-controlled synthesis of single-crystal silicon nanowires. Appl. Phys. Lett. 78(15), 2214–2216 (2001). https://doi.org/10.1063/1.1363692
A.K. Shalek, J.T. Robinson, E.S. Karp, J.S. Lee, D.-R. Ahn et al., Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proc. Natl. Acad. Sci. USA 107(5), 1870–1875 (2010). https://doi.org/10.1073/Proc.Natl.Acad.Sci.U.S.A.0909350107
Y.J. Hwang, C. Hahn, B. Liu, P. Yang, Photoelectrochemical properties of tio2 nanowire arrays: A study of the dependence on length and atomic layer deposition coating. ACS Nano 6(6), 5060–5069 (2012). https://doi.org/10.1021/nn300679d
S.M. George, Atomic layer deposition: An overview. Chem. Rev. 110(1), 111–131 (2010). https://doi.org/10.1021/cr900056b
H.J. Joyce, Q. Gao, H.H. Tan, C. Jagadish, Y. Kim et al., Iii-v semiconductor nanowires for optoelectronic device applications. Prog. Quant. Electron. 35(2–3), 23–75 (2011). https://doi.org/10.1016/j.pquantelec.2011.03.002
K. Ogata, K. Maejima, S. Fujita, S. Fujita, Growth mode control of ZnO toward nanorod structures or high-quality layered structures by metal-organic vapor phase epitaxy. J. Cryst. Growth 248, 25–30 (2003). https://doi.org/10.1016/S0022-0248(02)01843-2
D. Whang, S. Jin, C.M. Lieber, Nanolithography using hierarchically assembled nanowire masks. Nano Lett. 3(7), 951–954 (2003). https://doi.org/10.1021/nl034268a
T. Mårtensson, P. Carlberg, M. Borgström, L. Montelius, W. Seifert et al., Nanowire arrays defined by nanoimprint lithography. Nano Lett. 4(4), 699–702 (2004). https://doi.org/10.1021/nl035100s
R. Juhasz, N. Elfström, J. Linnros, Controlled fabrication of silicon nanowires by electron beam lithography and electrochemical size reduction. Nano Lett. 5(2), 275–280 (2005). https://doi.org/10.1021/nl0481573
Z. Huang, H. Fang, J. Zhu, Fabrication of silicon nanowire arrays with controlled diameter, length, and density. Adv. Mater. 19(5), 744–748 (2007). https://doi.org/10.1002/adma.200600892
A. del Campo, E. Arzt, Fabrication approaches for generating complex micro-and nanopatterns on polymeric surfaces. Chem. Rev. 108(3), 911–945 (2008). https://doi.org/10.1021/cr050018y
Y.Q. Fu, A. Colli, A. Fasoli, J.K. Luo, A.J. Flewitt et al., Deep reactive ion etching as a tool for nanostructure fabrication. J. Vac. Sci. Technol. B 27(3), 1520–1526 (2009). https://doi.org/10.1116/1.3065991
Z. Huang, N. Geyer, P. Werner, J. De Boor, U. Gösele, Metal-assisted chemical etching of silicon: A review: In memory of prof. Ulrich gösele. Adv. Mater. 23(2), 285–308 (2011). https://doi.org/10.1002/adma.201001784
H. Wang, M. Sun, K. Ding, M.T. Hill, C.-Z. Ning, A top-down approach to fabrication of high quality vertical heterostructure nanowire arrays. Nano Lett. 11(4), 1646–1650 (2011). https://doi.org/10.1021/nl2001132
O. Staufer, S. Weber, C.P. Bengtson, H. Bading, A. Rustom et al., Adhesion stabilized en masse intracellular electrical recordings from multicellular assemblies. Nano Lett. 19(5), 3244–3255 (2019). https://doi.org/10.1021/acs.nanolett.9b00784
X. Xie, A.M. Xu, M.R. Angle, N. Tayebi, P. Verma et al., Mechanical model of vertical nanowire cell penetration. Nano Lett. 13(12), 6002–6008 (2013). https://doi.org/10.1021/nl403201a
L. Hanson, Z.C. Lin, C. Xie, Y. Cui, B. Cui, Characterization of the cell–nanopillar interface by transmission electron microscopy. Nano Lett. 12(11), 5815–5820 (2012). https://doi.org/10.1021/nl303163y
A.M. Xu, A. Aalipour, S. Leal-Ortiz, A.H. Mekhdjian, X. Xie et al., Quantification of nanowire penetration into living cells. Nat. Commun. 5(1), 1–8 (2014). https://doi.org/10.1038/ncomms4613
M. Dipalo, A.F. McGuire, H.Y. Lou, V. Caprettini, G. Melle et al., Cells adhering to 3d vertical nanostructures: Cell membrane reshaping without stable internalization. Nano Lett. 18(9), 6100–6105 (2018). https://doi.org/10.1021/acs.nanolett.8b03163
B.D. Almquist, N.A. Melosh, Fusion of biomimetic stealth probes into lipid bilayer cores. Proc. Natl. Acad. Sci. USA 107(13), 5815–5820 (2010). https://doi.org/10.1073/Proc.Natl.Acad.Sci.U.S.A.0909250107
S.J. Luck, An Introduction to the Event-related Potential Technique (MIT Press; 2014)
B. Tian, C.M. Lieber, Nanowired bioelectric interfaces. Chem. Rev. 119(15), 9136–9152 (2019). https://doi.org/10.1021/acs.chemrev.8b00795
F. Pei, B. Tian, Nanoelectronics for minimally invasive cellular recordings. Adv. Funct. Mater. 1906210 (2019). https://doi.org/10.1002/adfm.201906210
G.W. Gross, A.N. Williams, J.H. Lucas, Recording of spontaneous activity with photoetched microelectrode surfaces from mouse spinal neurons in culture. J. Neurosci. Meth. 5(1–2), 13–22 (1982). https://doi.org/10.1016/0165-0270(82)90046-2
W.G. Regehr, J. Pine, C.S. Cohan, M.D. Mischke, D.W. Tank, Sealing cultured invertebrate neurons to embedded dish electrodes facilitates long-term stimulation and recording. J. Neurosci. Meth. 30(2), 91–106 (1989). https://doi.org/10.1016/0165-0270(89)90055-1
A.F. Johnstone, G.W. Gross, D.G. Weiss, O.H.-U. Schroeder, A. Gramowski et al., Microelectrode arrays: A physiologically based neurotoxicity testing platform for the 21st century. Neurotoxicology 31(4), 331–350 (2010). https://doi.org/10.1016/j.neuro.2010.04.001
L.R. Hochberg, M.D. Serruya, G.M. Friehs, J.A. Mukand, M. Saleh et al., Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442(7099), 164–171 (2006). https://doi.org/10.1038/nature04970
A. Berényi, Z. Somogyvári, A.J. Nagy, L. Roux, J.D. Long et al., Large-scale, high-density (up to 512 channels) recording of local circuits in behaving animals. J. Neurophysiol. 111(5), 1132–1149 (2014). https://doi.org/10.1152/jn.00785.2013
Y. Kubota, S. Yamagiwa, H. Sawahata, S. Idogawa, S. Tsuruhara et al., Long nanoneedle-electrode devices for extracellular and intracellular recording in vivo. Sens. Actuat. B Chem. 258, 1287–1294 (2018). https://doi.org/10.1016/j.snb.2017.11.152
M.J. Nelson, P. Pouget, E.A. Nilsen, C.D. Patten, J.D. Schall, Review of signal distortion through metal microelectrode recording circuits and filters. J. Neurosci. Meth. 169(1), 141–157 (2008). https://doi.org/10.1016/j.jneumeth.2007.12.010
P. Fromherz, Semiconductor chips with ion channels, nerve cells and brain. Physica E Low Dimens. Syst. Nanostruct. 16(1), 24–34 (2003). https://doi.org/10.1016/S1386-9477(02)00578-7
A. Cerea, V. Caprettini, G. Bruno, L. Lovato, G. Melle et al., De Angelis: selective intracellular delivery and intracellular recordings combined in mea biosensors. Lab Chip 18(22), 3492–3500 (2018). https://doi.org/10.1039/c8lc00435h
D.M. Bers, Cardiac excitation–contraction coupling. Nature 415(6868), 198–205 (2002). https://doi.org/10.1038/415198a
D.P. Zipes, J. Jalife, Cardiac Electrophysiology: From Cell to Bedside E-book: Expert Consult (Elsevier Health Sciences, 2009)
L.F. Santana, E.P. Cheng, W.J. Lederer, How does the shape of the cardiac action potential control calcium signaling and contraction in the heart? J. Mol. Cell Cardiol. 49(6), 901–903 (2010). https://doi.org/10.1016/j.yjmcc.2010.09.005
J.M. Nerbonne, R.S. Kass, Molecular physiology of cardiac repolarization. Physiol. Rev. 85(4), 1205–1253 (2005). https://doi.org/10.1152/physrev.00002.2005
P. Johnson, A. Freedberg, J. Marshall, Action of thyroid hormone on the transmembrane potentials from sinoatrial node cells and atrial muscle cells in isolated atria of rabbits. Cardiology 58(5), 273–289 (1973). https://doi.org/10.1159/000169643
D. DiFrancesco, Pacemaker mechanisms in cardiac tissue. Ann. Rev. Physiol. 55(1), 455–472 (1993). https://doi.org/10.1146/annurev.ph.55.030193.002323
E. Carmeliet, J. Vereecke, Adrenaline and the plateau phase of the cardiac action potential. Pflugers Arch. 313(4), 300–315 (1969). https://doi.org/10.1063/1.1753975
C.-H. Luo, Y. Rudy, A model of the ventricular cardiac action potential: Depolarization, repolarization, and their interaction. Circ. Res. 68(6), 1501–1526 (1991). https://doi.org/10.1161/01.RES.68.6.1501
Y. Rudy, Molecular basis of cardiac action potential repolarization. Ann. N. Y. Acad. Sci. 1123(1), 113–118 (2008). https://doi.org/10.1196/annals.1420.013
N. Shmoel, N. Rabieh, S.M. Ojovan, H. Erez, E. Maydan et al., Multisite electrophysiological recordings by self-assembled loose-patch-like junctions between cultured hippocampal neurons and mushroom-shaped microelectrodes. Sci. Rep. 6, 27110 (2016). https://doi.org/10.1038/srep27110
A.C. Dolphin, A. Lee, Presynaptic calcium channels: Specialized control of synaptic neurotransmitter release. Nat. Rev. Neurosci. 21(4), 213–229 (2020). https://doi.org/10.1038/s41583-020-0278-2
E.D. Adrian, Wedensky inhibition in relation to theall-or-none’principle in nerve. J. Physiol. 46(4–5), 384–412 (1913). https://doi.org/10.1113/jphysiol.1913.sp001598
E.D. Adrian, The Mechanism of Nervous Action, Electrical Studies of the Neurone (University of Pennsylvania Press; 2016)
G. Stuart, J. Schiller, B. Sakmann, Action potential initiation and propagation in rat neocortical pyramidal neurons. J. Physiol. 505(3), 617–632 (1997). https://doi.org/10.1111/j.1469-7793.1997.617ba.x
M. Dipalo, G.C. Messina, H. Amin, R. La Rocca, V. Shalabaeva et al., 3d plasmonic nanoantennas integrated with mea biosensors. Nanoscale 7(8), 3703–3711 (2015). https://doi.org/10.1039/c4nr05578k
G. Bruno, N. Colistra, G. Melle, A. Cerea, A. Hubarevich et al., Microfluidic multielectrode arrays for spatially localized drug delivery and electrical recordings of primary neuronal cultures. Front. Bioeng. Biotechnol. 8, 626 (2020). https://doi.org/10.3389/fbioe.2020.00626
A. Barbaglia, M. Dipalo, G. Melle, G. Iachetta, L. Deleye et al., Mirroring action potentials: Label-free, accurate, and noninvasive electrophysiological recordings of human-derived cardiomyocytes. Adv. Mater. 1, 2004234 (2021). https://doi.org/10.1002/adma.202004234
C. Chiappini, J.O. Martinez, E. De Rosa, C.S. Almeida, E. Tasciotti et al., Biodegradable nanoneedles for localized delivery of nanops in vivo: exploring the biointerface. ACS Nano 9(5), 5500–5509 (2015). https://doi.org/10.1021/acsnano.5b01490
Y. Cao, M. Hjort, H. Chen, F. Birey, S.A. Leal-Ortiz et al., Nondestructive nanostraw intracellular sampling for longitudinal cell monitoring. Proc. Natl. Acad. Sci. USA 114(10), E1866–E1874 (2017). https://doi.org/10.1073/pnas.1615375114
A.M. Xu, D.S. Wang, P. Shieh, Y. Cao, N.A. Melosh, Direct intracellular delivery of cell-impermeable probes of protein glycosylation by using nanostraws. ChemBioChem 18(7), 623–628 (2017). https://doi.org/10.1002/cbic.201600689