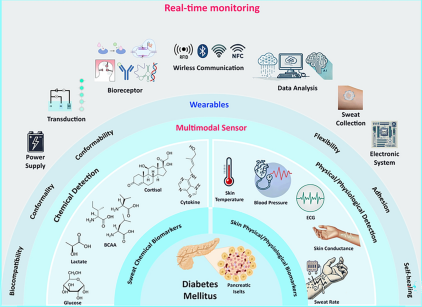
Noninvasive On-Skin Biosensors for Monitoring Diabetes Mellitus
Corresponding Author: Yi Li
Nano-Micro Letters,
Vol. 18 (2026), Article Number: 16
Abstract
Diabetes mellitus represents a major global health issue, driving the need for noninvasive alternatives to traditional blood glucose monitoring methods. Recent advancements in wearable technology have introduced skin-interfaced biosensors capable of analyzing sweat and skin biomarkers, providing innovative solutions for diabetes diagnosis and monitoring. This review comprehensively discusses the current developments in noninvasive wearable biosensors, emphasizing simultaneous detection of biochemical biomarkers (such as glucose, cortisol, lactate, branched-chain amino acids, and cytokines) and physiological signals (including heart rate, blood pressure, and sweat rate) for accurate, personalized diabetes management. We explore innovations in multimodal sensor design, materials science, biorecognition elements, and integration techniques, highlighting the importance of advanced data analytics, artificial intelligence-driven predictive algorithms, and closed-loop therapeutic systems. Additionally, the review addresses ongoing challenges in biomarker validation, sensor stability, user compliance, data privacy, and regulatory considerations. A holistic, multimodal approach enabled by these next-generation wearable biosensors holds significant potential for improving patient outcomes and facilitating proactive healthcare interventions in diabetes management.
Highlights:
1 A comprehensive and critical evaluation of recent advances in sweat-based biochemical and physiological biomarkers for noninvasive diabetes monitoring.
2 A novel emphasis on multimodal sensor integration—combining biochemical and physiological signals—to enhance accuracy, contextual awareness, and reliability in real-time diabetes management.
3 A forward-looking analysis of AI-driven biosensing systems, standardized protocols, and regulatory and ethical frameworks enabling autonomous, secure, and personalized diabetes care.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- A. Chaudhury, C. Duvoor, V.S.R. Dendi, S. Kraleti, A. Chada et al., Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front. Endocrinol. 8, 6 (2017). https://doi.org/10.3389/fendo.2017.00006
- H. Lee, Y.J. Hong, S. Baik, T. Hyeon, D.-H. Kim, Enzyme-based glucose sensor: from invasive to wearable device. Adv. Healthc. Mater. 7(8), 1701150 (2018). https://doi.org/10.1002/adhm.201701150
- A.I. Vinik, R.E. Maser, B.D. Mitchell, R. Freeman, Diabetic autonomic neuropathy. Diabetes Care 26(5), 1553–1579 (2003). https://doi.org/10.2337/diacare.26.5.1553
- Y. Marunaka, Roles of interstitial fluid pH in diabetes mellitus: glycolysis and mitochondrial function. World J. Diabetes 6(1), 125–135 (2015). https://doi.org/10.4239/wjd.v6.i1.125
- N.J. Rehrer, Fluid and electrolyte balance in ultra-endurance sport. Sports Med. 31(10), 701–715 (2001). https://doi.org/10.2165/00007256-200131100-00001
- K. Lian, H. Feng, S. Liu, K. Wang, Q. Liu et al., Insulin quantification towards early diagnosis of prediabetes/diabetes. Biosens. Bioelectron. 203, 114029 (2022). https://doi.org/10.1016/j.bios.2022.114029
- H. Han, Y. Cao, C. Feng, Y. Zheng, K. Dhana et al., Association of a healthy lifestyle with all-cause and cause-specific mortality among individuals with type 2 diabetes: a prospective study in UK biobank. Diabetes Care 45(2), 319–329 (2022). https://doi.org/10.2337/dc21-1512
- C.A. Whicher, S. O’Neill, R.I.G. Holt, Diabetes in the UK: 2019. Diabet. Med. 37(2), 242–247 (2020). https://doi.org/10.1111/dme.14225
- B.O. Roep, S. Thomaidou, R. van Tienhoven, A. Zaldumbide, Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 17(3), 150–161 (2021). https://doi.org/10.1038/s41574-020-00443-4
- K.L. Wolkowicz, E.M. Aiello, E. Vargas, H. Teymourian, F. Tehrani et al., A review of biomarkers in the context of type 1 diabetes: biological sensing for enhanced glucose control. Bioeng. Transl. Med. 6(2), e10201 (2021). https://doi.org/10.1002/btm2.10201
- A.L. McCall, Insulin therapy and hypoglycemia. Endocrinol. Metab. Clin. 41(1), 57–87 (2012). https://doi.org/10.1016/j.ecl.2012.03.001
- U. Galicia-Garcia, A. Benito-Vicente, S. Jebari, A. Larrea-Sebal, H. Siddiqi et al., Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 21(17), E6275 (2020). https://doi.org/10.3390/ijms21176275
- C. Ling, K. Bacos, T. Rönn, Epigenetics of type 2 diabetes mellitus and weight change: a tool for precision medicine? Nat. Rev. Endocrinol. 18(7), 433–448 (2022). https://doi.org/10.1038/s41574-022-00671-w
- D. Tomic, J.E. Shaw, D.J. Magliano, The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 18(9), 525–539 (2022). https://doi.org/10.1038/s41574-022-00690-7
- J.B. Cole, J.C. Florez, Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 16(7), 377–390 (2020). https://doi.org/10.1038/s41581-020-0278-5
- A. Fitero, S.G. Bungau, D.M. Tit, L. Endres, S.A. Khan et al., Comorbidities, associated diseases, and risk assessment in COVID-19: a systematic review. Int. J. Clin. Pract. 2022, 1571826 (2022). https://doi.org/10.1155/2022/1571826
- A.W. Stitt, T.M. Curtis, M. Chen, R.J. Medina, G.J. McKay et al., The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 51, 156–186 (2016). https://doi.org/10.1016/j.preteyeres.2015.08.001
- M. Kropp, O. Golubnitschaja, A. Mazurakova, L. Koklesova, N. Sargheini et al., Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications-risks and mitigation. EPMA J. 14(1), 21–42 (2023). https://doi.org/10.1007/s13167-023-00314-8
- J.L. Gross, M.J. de Azevedo, S.P. Silveiro, L.H. Canani, M.L. Caramori et al., Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28(1), 164–176 (2005). https://doi.org/10.2337/diacare.28.1.164
- M.K. Sulaiman, Diabetic nephropathy: recent advances in pathophysiology and challenges in dietary management. Diabetol. Metab. Syndr. 11(1), 7 (2019). https://doi.org/10.1186/s13098-019-0403-4
- E.L. Feldman, B.C. Callaghan, R. Pop-Busui, D.W. Zochodne, D.E. Wright et al., Diabetic neuropathy. Nat. Rev. Dis. Primers. 5, 41 (2019). https://doi.org/10.1038/s41572-019-0092-1
- W.J. Jeffcoate, K.G. Harding, Diabetic foot ulcers. Lancet 361(9368), 1545–1551 (2003). https://doi.org/10.1016/S0140-6736(03)13169-8
- L. Yazdanpanah, M. Nasiri, S. Adarvishi, Literature review on the management of diabetic foot ulcer. World J. Diabetes 6(1), 37–53 (2015). https://doi.org/10.4239/wjd.v6.i1.37
- H.E. Resnick, B.V. Howard, Diabetes and cardiovascular disease. Annu. Rev. Med. 53, 245–267 (2002). https://doi.org/10.1146/annurev.med.53.082901.103904
- J.R. Petrie, T.J. Guzik, R.M. Touyz, Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can. J. Cardiol. 34(5), 575–584 (2018). https://doi.org/10.1016/j.cjca.2017.12.005
- I. Idris, G.A. Thomson, J.C. Sharma, Diabetes mellitus and stroke. Int. J. Clin. Pract. 60(1), 48–56 (2006). https://doi.org/10.1111/j.1368-5031.2006.00682.x
- Z. Bloomgarden, R. Chilton, Diabetes and stroke: an important complication. J. Diabetes 13(3), 184–190 (2021). https://doi.org/10.1111/1753-0407.13142
- T. Thiruvoipati, C.E. Kielhorn, E.J. Armstrong, Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes. World J. Diabetes 6(7), 961–969 (2015). https://doi.org/10.4239/wjd.v6.i7.961
- S.P. Marso, W.R. Hiatt, Peripheral arterial disease in patients with diabetes. J. Am. Coll. Cardiol. 47(5), 921–929 (2006). https://doi.org/10.1016/j.jacc.2005.09.065
- L.C. Hofbauer, C.C. Brueck, S.K. Singh, H. Dobnig, Osteoporosis in patients with diabetes mellitus. J. Bone Miner. Res. 22(9), 1317–1328 (2007). https://doi.org/10.1359/jbmr.070510
- K. Wongdee, N. Charoenphandhu, Osteoporosis in diabetes mellitus: possible cellular and molecular mechanisms. World J. Diabetes 2(3), 41–48 (2011). https://doi.org/10.4239/wjd.v2.i3.41
- C. Sims-Robinson, B. Kim, A. Rosko, E.L. Feldman, How does diabetes accelerate Alzheimer disease pathology? Nat. Rev. Neurol. 6(10), 551–559 (2010). https://doi.org/10.1038/nrneurol.2010.130
- S. Pugazhenthi, L. Qin, P.H. Reddy, Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys. Acta BBA Mol. Basis Dis. 1863(5), 1037–1045 (2017). https://doi.org/10.1016/j.bbadis.2016.04.017
- M. Kumar, L. Mishra, R. Mohanty, R. Nayak, Diabetes and gum disease: the diabolic Duo. Diabetes Metab. Syndr. Clin. Res. Rev. 8(4), 255–258 (2014). https://doi.org/10.1016/j.dsx.2014.09.022
- I.L.C. Chapple, R. Genco, Working group 2 of the joint EFP/AAP workshop, Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP workshop on periodontitis and systemic diseases. J. Clin. Periodontol. 40(s14), S106–S112 (2013). https://doi.org/10.1111/jcpe.12077
- S.M. Danna, E. Graham, R.J. Burns, S.S. Deschênes, N. Schmitz, Association between depressive symptoms and cognitive function in persons with diabetes mellitus: a systematic review. PLoS ONE 11(8), e0160809 (2016). https://doi.org/10.1371/journal.pone.0160809
- S. Bellary, I. Kyrou, J.E. Brown, C.J. Bailey, Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat. Rev. Endocrinol. 17(9), 534–548 (2021). https://doi.org/10.1038/s41574-021-00512-2
- S. Klein, A. Gastaldelli, H. Yki-Järvinen, P.E. Scherer, Why does obesity cause diabetes? Cell Metab. 34(1), 11–20 (2022). https://doi.org/10.1016/j.cmet.2021.12.012
- X.R. Pan, G.W. Li, Y.H. Hu, J.X. Wang, W.Y. Yang et al., Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and diabetes study. Diabetes Care 20(4), 537–544 (1997). https://doi.org/10.2337/diacare.20.4.537
- J. Tuomilehto, J. Lindström, J.G. Eriksson, T.T. Valle, H. Hämäläinen et al., Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 344(18), 1343–1350 (2001). https://doi.org/10.1056/NEJM200105033441801
- W.C. Knowler, E. Barrett-Connor, S.E. Fowler, R.F. Hamman, J.M. Lachin et al., Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346(6), 393–403 (2002). https://doi.org/10.1056/NEJMoa012512
- J. Valabhji, E. Barron, D. Bradley, C. Bakhai, J. Fagg et al., Early outcomes from the English national health service diabetes prevention programme. Diabetes Care 43(1), 152–160 (2020). https://doi.org/10.2337/dc19-1425
- Y. Wang, C. Wang, K. Li, X. Song, X. Yan et al., Recent advances of nanomedicine-based strategies in diabetes and complications management: diagnostics, monitoring, and therapeutics. J. Control. Release 330, 618–640 (2021). https://doi.org/10.1016/j.jconrel.2021.01.002
- J.W. Stevens, K. Khunti, R. Harvey, M. Johnson, L. Preston et al., Preventing the progression to Type 2 diabetes mellitus in adults at high risk: a systematic review and network meta-analysis of lifestyle, pharmacological and surgical interventions. Diabetes Res. Clin. Pract. 107(3), 320–331 (2015). https://doi.org/10.1016/j.diabres.2015.01.027
- X. Li, X. Huang, J. Mo, H. Wang, Q. Huang et al., A fully integrated closed-loop system based on mesoporous microneedles-iontophoresis for diabetes treatment. Adv. Sci. 8(16), 2100827 (2021). https://doi.org/10.1002/advs.202100827
- Y. Liu, S. Zeng, W. Ji, H. Yao, L. Lin et al., Emerging theranostic nanomaterials in diabetes and its complications. Adv. Sci. 9(3), 2102466 (2022). https://doi.org/10.1002/advs.202102466
- S. Szunerits, S. Melinte, A. Barras, Q. Pagneux, A. Voronova et al., The impact of chemical engineering and technological advances on managing diabetes: present and future concepts. Chem. Soc. Rev. 50(3), 2102–2146 (2021). https://doi.org/10.1039/C9CS00886A
- J. Song, Y. Zhang, S.Y. Chan, Z. Du, Y. Yan et al., Hydrogel-based flexible materials for diabetes diagnosis, treatment, and management. NPJ Flex. Electron. 5, 26 (2021). https://doi.org/10.1038/s41528-021-00122-y
- S.A. Pullano, M. Greco, M.G. Bianco, D. Foti, A. Brunetti et al., Glucose biosensors in clinical practice: principles, limits and perspectives of currently used devices. Theranostics 12(2), 493–511 (2022). https://doi.org/10.7150/thno.64035
- W.D. Strain, S.V. Hope, A. Green, P. Kar, J. Valabhji et al., Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty. A national collaborative stakeholder initiative. Diabet. Med. 35(7), 838–845 (2018). https://doi.org/10.1111/dme.13644
- A.D. Association, 12. Older adults: Standards of medical care in diabetes-2020. Diabetes Care 43(Suppl 1), S152–S162 (2020). https://doi.org/10.2337/dc20-S012
- X. Lu, Q. Xie, X. Pan, R. Zhang, X. Zhang et al., Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 9(1), 262 (2024). https://doi.org/10.1038/s41392-024-01951-9
- F.H. Karlsson, V. Tremaroli, I. Nookaew, G. Bergström, C.J. Behre et al., Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498(7452), 99–103 (2013). https://doi.org/10.1038/nature12198
- W. Jia, J.C. Chan, T.Y. Wong, E.B. Fisher, Diabetes in China: epidemiology, pathophysiology and multi-omics. Nat. Metab. 7(1), 16–34 (2025). https://doi.org/10.1038/s42255-024-01190-w
- J.C.N. Chan, V. Malik, W. Jia, T. Kadowaki, C.S. Yajnik et al., Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 301(20), 2129–2140 (2009). https://doi.org/10.1001/jama.2009.726
- T.J. Lyons, A. Basu, Biomarkers in diabetes: hemoglobin A1c, vascular and tissue markers. Transl. Res. 159(4), 303–312 (2012). https://doi.org/10.1016/j.trsl.2012.01.009
- C. Guay, R. Regazzi, Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 9(9), 513–521 (2013). https://doi.org/10.1038/nrendo.2013.86
- M. Song, H. Bai, P. Zhang, X. Zhou, B. Ying, Promising applications of human-derived saliva biomarker testing in clinical diagnostics. Int. J. Oral Sci. 15, 2 (2023). https://doi.org/10.1038/s41368-022-00209-w
- H. de Puig, R.A. Lee, D. Najjar, X. Tan, L.R. Soeknsen et al., Minimally instrumented SHERLOCK (miSHERLOCK) for CRISPR-based point-of-care diagnosis of SARS-CoV-2 and emerging variants. Sci. Adv. 7(32), eabh2944 (2021). https://doi.org/10.1126/sciadv.abh2944
- J. Min, J. Tu, C. Xu, H. Lukas, S. Shin et al., Skin-interfaced wearable sweat sensors for precision medicine. Chem. Rev. 123(8), 5049–5138 (2023). https://doi.org/10.1021/acs.chemrev.2c00823
- C. Niederberger, A. Vermeersch, F. Davidhi, C.Y. Ewald, G. Havenith et al., Wearable sweat analysis to determine biological age. Trends Biotechnol. 41(9), 1113–1116 (2023). https://doi.org/10.1016/j.tibtech.2023.02.001
- Y. Zhu, S. Li, J. Li, N. Falcone, Q. Cui et al., Lab-on-a-contact lens: recent advances and future opportunities in diagnostics and therapeutics. Adv. Mater. 34(24), 2108389 (2022). https://doi.org/10.1002/adma.202108389
- K. Kim, H.J. Kim, H. Zhang, W. Park, D. Meyer et al., All-printed stretchable corneal sensor on soft contact lenses for noninvasive and painless ocular electrodiagnosis. Nat. Commun. 12(1), 1544 (2021). https://doi.org/10.1038/s41467-021-21916-8
- J. Heikenfeld, A. Jajack, B. Feldman, S.W. Granger, S. Gaitonde et al., Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37(4), 407–419 (2019). https://doi.org/10.1038/s41587-019-0040-3
- M. Friedel, I.A.P. Thompson, G. Kasting, R. Polsky, D. Cunningham et al., Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat. Biomed. Eng. 7(12), 1541–1555 (2023). https://doi.org/10.1038/s41551-022-00998-9
- H.C. Ates, C. Dincer, Wearable breath analysis. Nat. Rev. Bioeng. 1(2), 80–82 (2023). https://doi.org/10.1038/s44222-022-00011-7
- H.C. Ates, P.Q. Nguyen, L. Gonzalez-Macia, E. Morales-Narváez, F. Güder et al., End-to-end design of wearable sensors. Nat. Rev. Mater. 7(11), 887–907 (2022). https://doi.org/10.1038/s41578-022-00460-x
- M.C. Brothers, M. DeBrosse, C.C. Grigsby, R.R. Naik, S.M. Hussain et al., Achievements and challenges for real-time sensing of analytes in sweat within wearable platforms. Acc. Chem. Res. 52(2), 297–306 (2019). https://doi.org/10.1021/acs.accounts.8b00555
- L. Yang, H. Lv, M. Li, Y. Zhang, J. Liu et al., Multiple polarization effect of shell evolution on hierarchical hollow C@MnO2 composites and their wideband electromagnetic wave absorption properties. Chem. Eng. J. 392, 123666 (2020). https://doi.org/10.1016/j.cej.2019.123666
- E.P. Dutkiewicz, H.-Y. Chiu, P.L. Urban, Probing skin for metabolites and topical drugs with hydrogel micropatches. Anal. Chem. 89(5), 2664–2670 (2017). https://doi.org/10.1021/acs.analchem.6b04276
- D.P. Elpa, H.-Y. Chiu, S.-P. Wu, P.L. Urban, Skin metabolomics. Trends Endocrinol Metab 32, 66–75 (2021). https://doi.org/10.1016/j.tem.2020.11.009
- A.J. Thody, S. Shuster, Control and function of sebaceous glands. Physiol. Rev. 69(2), 383–416 (1989). https://doi.org/10.1152/physrev.1989.69.2.383
- J.R. Sempionatto, I. Jeerapan, S. Krishnan, J. Wang, Wearable chemical sensors: emerging systems for on-body analytical chemistry. Anal. Chem. 92(1), 378–396 (2020). https://doi.org/10.1021/acs.analchem.9b04668
- Y. Hu, C. Converse, M.C. Lyons, W.H. Hsu, Neural control of sweat secretion: a review. Br. J. Dermatol. 178(6), 1246–1256 (2018). https://doi.org/10.1111/bjd.15808
- P.A.J. Kolarsick, M.A. Kolarsick, C. Goodwin, Anatomy and physiology of the skin. J. Dermatol. Nurses’ Assoc. 3(4), 203–213 (2011). https://doi.org/10.1097/jdn.0b013e3182274a98
- M. Wang, Y. Yang, J. Min, Y. Song, J. Tu et al., A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6(11), 1225–1235 (2022). https://doi.org/10.1038/s41551-022-00916-z
- L.B. Baker, Physiology of sweat gland function: the roles of sweating and sweat composition in human health. Temperature 6(3), 211–259 (2019). https://doi.org/10.1080/23328940.2019.1632145
- Y. Zhang, Y. Chen, J. Huang, Y. Liu, J. Peng et al., Skin-interfaced microfluidic devices with one-opening chambers and hydrophobic valves for sweat collection and analysis. Lab Chip 20(15), 2635–2645 (2020). https://doi.org/10.1039/D0LC00400F
- J. Choi, D. Kang, S. Han, S.B. Kim, J.A. Rogers, Thin, soft, skin-mounted microfluidic networks with capillary bursting valves for chrono-sampling of sweat. Adv. Healthc. Mater. 6(5), 1601355 (2017). https://doi.org/10.1002/adhm.201601355
- H. Shi, Y. Cao, Y. Zeng, Y. Zhou, W. Wen et al., Wearable tesla valve-based sweat collection device for sweat colorimetric analysis. Talanta 240, 123208 (2022). https://doi.org/10.1016/j.talanta.2022.123208
- J. Son, G.Y. Bae, S. Lee, G. Lee, S.W. Kim et al., Cactus-spine-inspired sweat-collecting patch for fast and continuous monitoring of sweat. Adv. Mater. 33(40), e2102740 (2021). https://doi.org/10.1002/adma.202102740
- L. Wang, T. Xu, X. He, X. Zhang, Flexible, self-healable, adhesive and wearable hydrogel patch for colorimetric sweat detection. J. Mater. Chem. C 9(41), 14938–14945 (2021). https://doi.org/10.1039/d1tc03905a
- B. Dai, K. Li, L. Shi, X. Wan, X. Liu et al., Bioinspired Janus textile with conical micropores for human body moisture and thermal management. Adv. Mater. 31(41), 1904113 (2019). https://doi.org/10.1002/adma.201904113
- M.A. Yokus, M.A. Daniele, Integrated non-invasive biochemical and biophysical sensing systems for health and performance monitoring: a systems perspective. Biosens. Bioelectron. 184, 113249 (2021). https://doi.org/10.1016/j.bios.2021.113249
- A. Hauke, P. Simmers, Y.R. Ojha, B.D. Cameron, R. Ballweg et al., Complete validation of a continuous and blood-correlated sweat biosensing device with integrated sweat stimulation. Lab Chip 18(24), 3750–3759 (2018). https://doi.org/10.1039/C8LC01082J
- T. Saha, S. Mukherjee, M.D. Dickey, O.D. Velev, Harvesting and manipulating sweat and interstitial fluid in microfluidic devices. Lab Chip 24(5), 1244–1265 (2024). https://doi.org/10.1039/D3LC00874F
- M.J. Patterson, S.D.R. Galloway, M.A. Nimmo, Variations in regional sweat composition in normal human males. Exp. Physiol. 85(6), 869–875 (2000). https://doi.org/10.1017/s0958067000020583
- P.J. Derbyshire, H. Barr, F. Davis, S.P.J. Higson, Lactate in human sweat: a critical review of research to the present day. J. Physiol. Sci. 62(6), 429–440 (2012). https://doi.org/10.1007/s12576-012-0213-z
- M.J. Buono, N.V.L. Lee, P.W. Miller, The relationship between exercise intensity and the sweat lactate excretion rate. J. Physiol. Sci. 60(2), 103–107 (2010). https://doi.org/10.1007/s12576-009-0073-3
- M.C.G.J. Brouwers, J.C. Ham, E. Wisse, S. Misra, S. Landewe et al., Elevated lactate levels in patients with poorly regulated type 1 diabetes and glycogenic hepatopathy: a new feature of Mauriac syndrome. Diabetes Care 38(2), e11–e12 (2015). https://doi.org/10.2337/dc14-2205
- K. Van Hoovels, X. Xuan, M. Cuartero, M. Gijssel, M. Swarén et al., Can wearable sweat lactate sensors contribute to sports physiology? ACS Sens. 6(10), 3496–3508 (2021). https://doi.org/10.1021/acssensors.1c01403
- J. Moyer, D. Wilson, I. Finkelshtein, B. Wong, R. Potts, Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 14(5), 398–402 (2012). https://doi.org/10.1089/dia.2011.0262
- K. Sakaguchi, Y. Hirota, N. Hashimoto, W. Ogawa, T. Hamaguchi et al., Evaluation of a minimally invasive system for measuring glucose area under the curve during oral glucose tolerance tests: usefulness of sweat monitoring for precise measurement. J. Diabetes Sci. Technol. 7(3), 678–688 (2013). https://doi.org/10.1177/193229681300700313
- A. Sedighi, M. Montazer, S. Mazinani, Synthesis of wearable and flexible NiP0.1-SnOx/PANI/CuO/cotton towards a non-enzymatic glucose sensor. Biosens. Bioelectron. 135, 192–199 (2019). https://doi.org/10.1016/j.bios.2019.04.010
- Y. Pan, R. Yu, Y. Jiang, H. Zhong, Q. Yuan et al., Heterogeneous CuxO nano-skeletons from waste electronics for enhanced glucose detection. Nano-Micro Lett. 16(1), 249 (2024). https://doi.org/10.1007/s40820-024-01467-5
- C.J. Harvey, R.F. LeBouf, A.B. Stefaniak, Formulation and stability of a novel artificial human sweat under conditions of storage and use. Toxicol. Vitro 24(6), 1790–1796 (2010). https://doi.org/10.1016/j.tiv.2010.06.016
- S. Wang, A. Zhao, G. Li, X. Sun, J. Wang et al., In situ regenerable molecularly imprinted polymer biosensor for electrochemical detection of nonelectroactive branched-chain amino acids in human sweat. Anal. Chem. 96(51), 20287–20295 (2024). https://doi.org/10.1021/acs.analchem.4c05144
- G. Cizza, A.H. Marques, F. Eskandari, I.C. Christie, S. Torvik et al., Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: the POWER study. Biol. Psychiatry 64(10), 907–911 (2008). https://doi.org/10.1016/j.biopsych.2008.05.035
- C. Huang, W. Yang, H. Wang, S. Huang, S. Gao et al., Flexible/regenerative nanosensor with automatic sweat collection for cytokine storm biomarker detection. ACS Nano 18(32), 21198–21210 (2024). https://doi.org/10.1021/acsnano.4c04456
- B. Wang, C. Zhao, Z. Wang, K.-A. Yang, X. Cheng et al., Wearable aptamer-field-effect transistor sensing system for noninvasive Cortisol monitoring. Sci. Adv. 8(1), eabk0967 (2022). https://doi.org/10.1126/sciadv.abk0967
- R.M. Torrente-Rodríguez, J. Tu, Y. Yang, J. Min, M. Wang et al., Investigation of Cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2(4), 921–937 (2020). https://doi.org/10.1016/j.matt.2020.01.021
- I. Chiodini, G. Adda, A. Scillitani, F. Coletti, V. Morelli et al., Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care 30(1), 83–88 (2007). https://doi.org/10.2337/dc06-1267
- J. Ok, S. Park, Y.H. Jung, T.I. Kim, Wearable and implantable Cortisol-sensing electronics for stress monitoring. Adv. Mater. 36(1), e2211595 (2024). https://doi.org/10.1002/adma.202211595
- M.S. Rahman, K.S. Hossain, S. Das, S. Kundu, E.O. Adegoke et al., Role of insulin in health and disease: an update. Int. J. Mol. Sci. 22(12), 6403 (2021). https://doi.org/10.3390/ijms22126403
- P.J. Hantzidiamantis, S.L. Lappin, Physiology, glucose. (2019).
- M. Tesauro, F.A. Mazzotta, Chapter 3—Pathophysiology of diabetes, in Transplantation, bioengineering, and regeneration of the endocrine pancreas. ed. by G. Orlando, L. Piemonti, C. Ricordi, R.J. Stratta, R.W. Gruessner (Elsevier, Hoboken, 2020), pp.37–47. https://doi.org/10.1016/B978-0-12-814833-4.00003-4
- S.E. Kahn, M.E. Cooper, S. Del Prato, Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383(9922), 1068–1083 (2014). https://doi.org/10.1016/S0140-6736(13)62154-6
- R.J. Wright, B.M. Frier, Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab. Res. Rev. 24(5), 353–363 (2008). https://doi.org/10.1002/dmrr.865
- R.M. Sapolsky, Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress 1(1), 1–19 (1996). https://doi.org/10.3109/10253899609001092
- S. Vaddiraju, D.J. Burgess, I. Tomazos, F.C. Jain, F. Papadimitrakopoulos, Technologies for continuous glucose monitoring: current problems and future promises. J. Diabetes Sci. Technol. 4(6), 1540–1562 (2010). https://doi.org/10.1177/193229681000400632
- S. Emaminejad, W. Gao, E. Wu, Z.A. Davies, H. Yin Nyein et al., Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. U.S.A. 114(18), 4625–4630 (2017). https://doi.org/10.1073/pnas.1701740114
- J.R. Sempionatto, J.-M. Moon, J. Wang, Touch-based fingertip blood-free reliable glucose monitoring: personalized data processing for predicting blood glucose concentrations. ACS Sens. 6(5), 1875–1883 (2021). https://doi.org/10.1021/acssensors.1c00139
- E.V. Karpova, E.V. Shcherbacheva, A.A. Galushin, D.V. Vokhmyanina, E.E. Karyakina et al., Noninvasive diabetes monitoring through continuous analysis of sweat using flow-through glucose biosensor. Anal. Chem. 91(6), 3778–3783 (2019). https://doi.org/10.1021/acs.analchem.8b05928
- H.Y.Y. Nyein, M. Bariya, L. Kivimäki, S. Uusitalo, T.S. Liaw et al., Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 5(8), eaaw9906 (2019). https://doi.org/10.1126/sciadv.aaw9906
- L. Klous, C.J. de Ruiter, S. Scherrer, N. Gerrett, H.A.M. Daanen, The (in)dependency of blood and sweat sodium, chloride, potassium, ammonia, lactate and glucose concentrations during submaximal exercise. Eur. J. Appl. Physiol. 121(3), 803–816 (2021). https://doi.org/10.1007/s00421-020-04562-8
- N. Davis, J. Heikenfeld, C. Milla, A. Javey, The challenges and promise of sweat sensing. Nat. Biotechnol. 42(6), 860–871 (2024). https://doi.org/10.1038/s41587-023-02059-1
- J.D. Rabinowitz, S. Enerbäck, Lactate: the ugly duckling of energy metabolism. Nat. Metab. 2(7), 566–571 (2020). https://doi.org/10.1038/s42255-020-0243-4
- I. San-Millán, G.A. Brooks, Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 38(2), 119–133 (2017). https://doi.org/10.1093/carcin/bgw127
- M. Adeva-Andany, M. López-Ojén, R. Funcasta-Calderón, E. Ameneiros-Rodríguez, C. Donapetry-García et al., Comprehensive review on lactate metabolism in human health. Mitochondrion 17, 76–100 (2014). https://doi.org/10.1016/j.mito.2014.05.007
- V. Qvisth, E. Hagström-Toft, E. Moberg, S. Sjöberg, J. Bolinder, Lactate release from adipose tissue and skeletal muscle in vivo: defective insulin regulation in insulin-resistant obese women. Am. J. Physiol. Endocrinol. Metab. 292(3), E709–E714 (2007). https://doi.org/10.1152/ajpendo.00104.2006
- S.O. Crawford, R.C. Hoogeveen, F.L. Brancati, B.C. Astor, C.M. Ballantyne et al., Association of blood lactate with type 2 diabetes: the Atherosclerosis Risk in Communities Carotid MRI Study. Int. J. Epidemiol. 39(6), 1647–1655 (2010). https://doi.org/10.1093/ije/dyq126
- L. Metz, P. Sirvent, G. Py, J.-F. Brun, C. Fédou et al., Relationship between blood lactate concentration and substrate utilization during exercise in type 2 diabetic postmenopausal women. Metabolism 54(8), 1102–1107 (2005). https://doi.org/10.1016/j.metabol.2005.03.015
- F. Berhane, A. Fite, N. Daboul, W. Al-Janabi, Z. Msallaty et al., Plasma lactate levels increase during hyperinsulinemic euglycemic clamp and oral glucose tolerance test. J. Diabetes Res. 2015, 102054 (2015). https://doi.org/10.1155/2015/102054
- E.V. Karpova, A.I. Laptev, E.A. Andreev, E.E. Karyakina, A.A. Karyakin, Relationship between sweat and blood lactate levels during exhaustive physical exercise. ChemElectroChem 7(1), 191–194 (2020). https://doi.org/10.1002/celc.201901703
- A. Márquez, J. Aymerich, M. Dei, R. Rodríguez-Rodríguez, M. Vázquez-Carrera et al., Reconfigurable multiplexed point of care system for monitoring type 1 diabetes patients. Biosens. Bioelectron. 136, 38–46 (2019). https://doi.org/10.1016/j.bios.2019.04.015
- N.A. Taylor, C.A. Machado-Moreira, Regional variations in transepidermal water loss, eccrine sweat gland density, sweat secretion rates and electrolyte composition in resting and exercising humans. Extreme Physiol. Med. 2(1), 4 (2013). https://doi.org/10.1186/2046-7648-2-4
- C.B. Newgard, J. An, J.R. Bain, M.J. Muehlbauer, R.D. Stevens et al., A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9(4), 311–326 (2009). https://doi.org/10.1016/j.cmet.2009.02.002
- X. Chen, W. Yang, Branched-chain amino acids and the association with type 2 diabetes. J. Diabetes Investig. 6(4), 369–370 (2015). https://doi.org/10.1111/jdi.12345
- T.J. Wang, M.G. Larson, R.S. Vasan, S. Cheng, E.P. Rhee et al., Metabolite profiles and the risk of developing diabetes. Nat. Med. 17(4), 448–453 (2011). https://doi.org/10.1038/nm.2307
- M. Jaromy, J.D. Miller, Potential clinical applications for continuous ketone monitoring in the hospitalized patient with diabetes. Curr. Diab. Rep. 22(10), 501–510 (2022). https://doi.org/10.1007/s11892-022-01489-6
- V.A. Fonseca, M.A. Haggar, Achieving glycaemic targets with basal insulin in T2DM by individualizing treatment. Nat. Rev. Endocrinol. 10(5), 276–281 (2014). https://doi.org/10.1038/nrendo.2014.17
- F. Vanweert, M. de Ligt, J. Hoeks, M.K.C. Hesselink, P. Schrauwen et al., Elevated plasma branched-chain amino acid levels correlate with type 2 diabetes-related metabolic disturbances. J. Clin. Endocrinol. Metab. 106(4), e1827–e1836 (2021). https://doi.org/10.1210/clinem/dgaa751
- H. Nakamura, H. Jinzu, K. Nagao, Y. Noguchi, N. Shimba et al., Plasma amino acid profiles are associated with insulin, C-peptide and adiponectin levels in type 2 diabetic patients. Nutr. Diabetes 4(9), e133 (2014). https://doi.org/10.1038/nutd.2014.32
- Y. Zheng, Y. Li, Q. Qi, A. Hruby, J.E. Manson et al., Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int. J. Epidemiol. 45(5), 1482–1492 (2016). https://doi.org/10.1093/ije/dyw143
- A. Ray, Cytokines and their role in health and disease: a brief overview. MOJ Immunol. 4(2), 00121 (2016). https://doi.org/10.15406/moji.2016.04.00121
- S. Tsalamandris, A.S. Antonopoulos, E. Oikonomou, G.-A. Papamikroulis, G. Vogiatzi et al., The role of inflammation in diabetes: current concepts and future perspectives. Eur. Cardiol. 14(1), 50–59 (2019). https://doi.org/10.15420/ecr.2018.33.1
- V. Wieser, A.R. Moschen, H. Tilg, Inflammation, cytokines and insulin resistance: a clinical perspective. Arch. Immunol. Ther. Exp. 61(2), 119–125 (2013). https://doi.org/10.1007/s00005-012-0210-1
- A. Rabinovitch, W.L. Suarez-Pinzon, Role of cytokines in the pathogenesis of autoimmune diabetes mellitus. Rev. Endocr. Metab. Dis. 4, 291–299 (2003). https://doi.org/10.1023/A:1025160614313
- Q. Li, B. Xu, S.A. Michie, K.H. Rubins, R.D. Schreriber et al., Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc. Natl. Acad. Sci. U.S.A. 105(34), 12439–12444 (2008). https://doi.org/10.1073/pnas.0806439105
- H. Thomas, J. Trapani, T. Kay, The role of perforin and granzymes in diabetes. Cell Death Differ. 17, 577–585 (2010). https://doi.org/10.1038/cdd.2009.165
- P. Marques-Vidal, F. Bastardot, R. von Känel, F. Paccaud, M. Preisig et al., Association between circulating cytokine levels, diabetes and insulin resistance in a population-based sample (CoLaus study). Clin. Endocrinol. 78(2), 232–241 (2013). https://doi.org/10.1111/j.1365-2265.2012.04384.x
- I. Hameed, S.R. Masoodi, S.A. Mir, M. Nabi, K. Ghazanfar et al., Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J. Diabetes 6(4), 598–612 (2015). https://doi.org/10.4239/wjd.v6.i4.598
- A. Marques-Deak, G. Cizza, F. Eskandari, S. Torvik, I.C. Christie et al., Measurement of cytokines in sweat patches and plasma in healthy women: validation in a controlled study. J. Immunol. Methods 315(1–2), 99–109 (2006). https://doi.org/10.1016/j.jim.2006.07.011
- V. Syngle, A. Syngle, N. Garg, P. Krishan, I. Verma, Predictors of autonomic neuropathy in rheumatoid arthritis. Auton. Neurosci. 201, 54–59 (2016). https://doi.org/10.1016/j.autneu.2016.07.008
- T. Kuo, A. McQueen, T.-C. Chen, J.-C. Wang, Regulation of glucose homeostasis by glucocorticoids. Glucocorticoid Signaling (Springer, New York, 2015), pp.99–126. https://doi.org/10.1007/978-1-4939-2895-8_5
- P.H. Black, The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med. Hypotheses 67(4), 879–891 (2006). https://doi.org/10.1016/j.mehy.2006.04.008
- P. Anagnostis, V.G. Athyros, K. Tziomalos, A. Karagiannis, D.P. Mikhailidis, The pathogenetic role of Cortisol in the metabolic syndrome: a hypothesis. J. Clin. Endocrinol. Metab. 94(8), 2692–2701 (2009). https://doi.org/10.1210/jc.2009-0370
- S. Khani, J.A. Tayek, Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome. Clin. Sci. 101(6), 739–747 (2001). https://doi.org/10.1042/cs1010739
- P.R. Bratusch-Marrain, Insulin-counteracting hormones: their impact on glucose metabolism. Diabetologia 24(2), 74–79 (1983). https://doi.org/10.1007/BF00297384
- R.A. Hackett, A. Steptoe, Type 2 diabetes mellitus and psychological stress—a modifiable risk factor. Nat. Rev. Endocrinol. 13(9), 547–560 (2017). https://doi.org/10.1038/nrendo.2017.64
- P. Pearlmutter, G. DeRose, C. Samson, N. Linehan, Y. Cen et al., Sweat and saliva Cortisol response to stress and nutrition factors. Sci. Rep. 10(1), 19050 (2020). https://doi.org/10.1038/s41598-020-75871-3
- E. Russell, G. Koren, M. Rieder, S.H.M. Van Uum, The detection of Cortisol in human sweat: implications for measurement of Cortisol in hair. Ther. Drug Monit. 36(1), 30–34 (2014). https://doi.org/10.1097/FTD.0b013e31829daa0a
- D. Shabeeb, M. Najafi, G. Hasanzadeh, M.R. Hadian, A.E. Musa et al., Electrophysiological measurements of diabetic peripheral neuropathy: a systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 12(4), 591–600 (2018). https://doi.org/10.1016/j.dsx.2018.03.026
- P. Novak, Electrochemical skin conductance: a systematic review. Clin. Auton. Res. 29(1), 17–29 (2019). https://doi.org/10.1007/s10286-017-0467-x
- C.M. Casellini, H.K. Parson, M.S. Richardson, M.L. Nevoret, A.I. Vinik, Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol. Ther. 15(11), 948–953 (2013). https://doi.org/10.1089/dia.2013.0129
- J.D. England, G.S. Gronseth, G. Franklin, R.G. Miller, A.K. Asbury et al., Distal symmetric polyneuropathy: a definition for clinical research: report of the American academy of neurology, the American association of electrodiagnostic medicine, and the American academy of physical medicine and rehabilitation. Neurology 64(2), 199–207 (2005). https://doi.org/10.1212/01.WNL.0000149522.32823.EA
- A. Hovaguimian, C.H. Gibbons, Diagnosis and treatment of pain in small-fiber neuropathy. Curr. Pain Headache Rep. 15(3), 193–200 (2011). https://doi.org/10.1007/s11916-011-0181-7
- B.I. Freedman, S.C. Smith, B.M. Bagwell, J. Xu, D.W. Bowden et al., Electrochemical skin conductance in diabetic kidney disease. Am. J. Nephrol. 41(6), 438–447 (2015). https://doi.org/10.1159/000437342
- S. Kim, J. Cho, B. Ku, M. Jun, G. Kim et al., Variability of electrochemical skin conductance for screening diabetes mellitus. Biomed. Eng. Lett. 9(2), 267–274 (2019). https://doi.org/10.1007/s13534-019-00111-1
- T. He, C. Wang, A. Zuo, P. Liu, R. Zhao et al., Electrochemical skin conductance may be used to screen for diabetic cardiac autonomic neuropathy in a Chinese population with diabetes. J. Diabetes Res. 2017(1), 8289740 (2017). https://doi.org/10.1155/2017/8289740
- Y.-R. Lai, C.-C. Huang, B.-C. Cheng, N.-W. Tsai, W.-C. Chiu et al., Feasibility of combining heart rate variability and electrochemical skin conductance as screening and severity evaluation of cardiovascular autonomic neuropathy in type 2 diabetes. J. Diabetes Investig. 12(9), 1671–1679 (2021). https://doi.org/10.1111/jdi.13518
- Y.-R. Lai, C.-C. Huang, W.-C. Chiu, B.-C. Cheng, T.-Y. Lin et al., Predictive value of heart rate variability and electrochemical skin conductance measurements for cardiovascular autonomic neuropathy persistence in type 2 diabetes and prediabetes: a 3-year follow-up study. Neurophysiol. Clin. 54(3), 102946 (2024). https://doi.org/10.1016/j.neucli.2024.102946
- K. Mohammedi, M. Woodward, Y. Hirakawa, S. Zoungas, B. Williams et al., Microvascular and macrovascular disease and risk for major peripheral arterial disease in patients with type 2 diabetes. Diabetes Care 39(10), 1796–1803 (2016). https://doi.org/10.2337/dc16-0588
- M.J. Fowler, Microvascular and macrovascular complications of diabetes. Clin. Diabetes 29(3), 116–122 (2011). https://doi.org/10.2337/diaclin.29.3.116
- T. Shinjo, F. Nishimura, The bidirectional association between diabetes and periodontitis, from basic to clinical. Jpn. Dent. Sci. Rev. 60, 15–21 (2024). https://doi.org/10.1016/j.jdsr.2023.12.002
- H. Kitaoka, M. Majima, A. Kitazawa, S. Sakane, K. Takeda et al., Impaired regulation of skin temperature in patients with diabetes mellitus evaluated by the cold exposure test. Bull. Osaka Med. Coll. 35(1–2), 99–105 (1989)
- J. Petrofsky, L. Berk, F. Alshammari, H. Lee, A. Hamdan et al., The effect of moist air on skin blood flow and temperature in subjects with and without diabetes. Diabetes Technol. Ther. 14(2), 105–116 (2012). https://doi.org/10.1089/dia.2011.0128
- S. Bagavathiappan, J. Philip, T. Jayakumar, B. Raj, P.N. Rao et al., Correlation between plantar foot temperature and diabetic neuropathy: a case study by using an infrared thermal imaging technique. J. Diabetes Sci. Technol. 4(6), 1386–1392 (2010). https://doi.org/10.1177/193229681000400613
- V.J. Houghton, V.M. Bower, D.C. Chant, Is an increase in skin temperature predictive of neuropathic foot ulceration in people with diabetes? A systematic review and meta-analysis. J. Foot Ankle Res. 6(1), 31 (2013). https://doi.org/10.1186/1757-1146-6-31
- J.J. van Netten, M. Prijs, J.G. van Baal, C. Liu, F. van der Heijden et al., Diagnostic values for skin temperature assessment to detect diabetes-related foot complications. Diabetes Technol. Ther. 16(11), 714–721 (2014). https://doi.org/10.1089/dia.2014.0052
- A. Berbudi, N. Rahmadika, A.I. Tjahjadi, R. Ruslami, Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 16(5), 442–449 (2020). https://doi.org/10.2174/1573399815666191024085838
- L. Korbel, J.D. Spencer, Diabetes mellitus and infection: an evaluation of hospital utilization and management costs in the United States. J. Diabetes Complicat. 29(2), 192–195 (2015). https://doi.org/10.1016/j.jdiacomp.2014.11.005
- T.V. Rohm, D.T. Meier, J.M. Olefsky, M.Y. Donath, Inflammation in obesity, diabetes, and related disorders. Immunity 55(1), 31–55 (2022). https://doi.org/10.1016/j.immuni.2021.12.013
- B.J. Sparks-DeFriese, Chapter 29—Vascular ulcers, in Physical rehabilitation. ed. by M.H. Cameron, L.G. Monroe (W.B. Saunders, Saint Louis, 2007), pp.777–802. https://doi.org/10.1016/B978-072160361-2.50032-6
- F. Andrasik, C. Rime, Chapter 130—Biofeedback, in Pain management (second edition). ed. by S.D. Waldman (W.B. Saunders, Philadelphia, 2011), pp.954–962. https://doi.org/10.1016/B978-1-4377-0721-2.00130-6
- S. Singaram, K. Ramakrishnan, J. Selvam, M. Senthil, V. Narayanamurthy, Sweat gland morphology and physiology in diabetes, neuropathy, and nephropathy: a review. Arch. Physiol. Biochem. 130(4), 437–451 (2024). https://doi.org/10.1080/13813455.2022.2114499
- T. Schlereth, M. Dieterich, F. Birklein, Hyperhidrosis: causes and treatment of enhanced sweating. Dtsch. Arztebl. Int. 106(3), 32–37 (2009). https://doi.org/10.3238/arztebl.2009.0032
- B.M.W. Illigens, C.H. Gibbons, Sweat testing to evaluate autonomic function. Clin. Auton. Res. 19(2), 79–87 (2009). https://doi.org/10.1007/s10286-008-0506-8
- V. Provitera, M. Nolano, G. Caporaso, A. Stancanelli, L. Santoro et al., Evaluation of sudomotor function in diabetes using the dynamic sweat test. Neurology 74(1), 50–56 (2010). https://doi.org/10.1212/WNL.0b013e3181c7da4b
- W.R. Kennedy, M. Sakuta, D. Sutherland, F.C. Goetz, Quantitation of the sweating deficiency in diabetes mellitus. Ann. Neurol. 15(5), 482–488 (1984). https://doi.org/10.1002/ana.410150514
- M. Asahina, Y. Yamanaka, Y. Akaogi, S. Kuwabara, Y. Koyama et al., Measurements of sweat response and skin vasomotor reflex for assessment of autonomic dysfunction in patients with diabetes. J. Diabetes Complicat. 22(4), 278–283 (2008). https://doi.org/10.1016/j.jdiacomp.2007.03.009
- S. Stern, S. Sclarowsky, The ECG in diabetes mellitus. Circulation 120(16), 1633–1636 (2009). https://doi.org/10.1161/circulationaha.109.897496
- J.J. McMurray, H. Uno, P. Jarolim, A.S. Desai, D. de Zeeuw et al., Predictors of fatal and nonfatal cardiovascular events in patients with type 2 diabetes mellitus, chronic kidney disease, and Anemia: an analysis of the Trial to Reduce cardiovascular Events with Aranesp (darbepoetin-Alfa) Therapy (TREAT). Am. Heart J. 162(4), 748-755.e3 (2011). https://doi.org/10.1016/j.ahj.2011.07.016
- B.S. Rana, M.M. Band, S. Ogston, A.D. Morris, S.D. Pringle et al., Relation of QT interval dispersion to the number of different cardiac abnormalities in diabetes mellitus. Am. J. Cardiol. 90(5), 483–487 (2002). https://doi.org/10.1016/s0002-9149(02)02518-3
- V.S. Gokhale, M.P. Jeyaseelan, Detailed ECG analysis in type 2 diabetes mellitus: a predictor of multitude of complications. Int. J. Res. Med. Sci. 8(3), 1030 (2020). https://doi.org/10.18203/2320-6012.ijrms20200775
- B.M. Leon, Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 6(13), 1246 (2015). https://doi.org/10.4239/wjd.v6.i13.1246
- J.R. Sowers, M. Epstein, E.D. Frohlich, Diabetes, hypertension, and cardiovascular disease. Hypertension 37(4), 1053–1059 (2001). https://doi.org/10.1161/01.hyp.37.4.1053
- P. Lopez-Jaramillo, J. Lopez-Lopez, C. Lopez-Lopez, M.I. Rodriguez-Alvarez, The goal of blood pressure in the hypertensive patient with diabetes is defined: now the challenge is go from recommendations to practice. Diabetol. Metab. Syndr. 6(1), 31 (2014). https://doi.org/10.1186/1758-5996-6-31
- P. Passarella, T.A. Kiseleva, F.V. Valeeva, A.R. Gosmanov, Hypertension management in diabetes: 2018 update. Diabetes Spectr. 31(3), 218–224 (2018). https://doi.org/10.2337/ds17-0085
- G.L. Bakris, The importance of blood pressure control in the patient with diabetes. Am. J. Med. 116(5), 30–38 (2004). https://doi.org/10.1016/j.amjmed.2003.10.018
- C.A. Emdin, K. Rahimi, B. Neal, T. Callender, V. Perkovic et al., Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 313(6), 603–615 (2015). https://doi.org/10.1001/jama.2014.18574
- M.A. Hill, Y. Yang, L. Zhang, Z. Sun, G. Jia et al., Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 119, 154766 (2021). https://doi.org/10.1016/j.metabol.2021.154766
- G. Jia, J.R. Sowers, Hypertension in diabetes: an update of basic mechanisms and clinical disease. Hypertension 78(5), 1197–1205 (2021). https://doi.org/10.1161/HYPERTENSIONAHA.121.17981
- A.A. da Silva, J.M. do Carmo, X. Li, Z. Wang, A.J. Mouton et al., Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Can. J. Cardiol. 36(5), 671–682 (2020). https://doi.org/10.1016/j.cjca.2020.02.066
- E. Ferrannini, W.C. Cushman, Diabetes and hypertension: the bad companions. Lancet 380(9841), 601–610 (2012). https://doi.org/10.1016/S0140-6736(12)60987-8
- K. Pickwell, V. Siersma, M. Kars, J. Apelqvist, K. Bakker et al., Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care 38(5), 852–857 (2015). https://doi.org/10.2337/dc14-1598
- J.L. Edwards, A.M. Vincent, H.T. Cheng, E.L. Feldman, Diabetic neuropathy: mechanisms to management. Pharmacol. Ther. 120, 1–34 (2008). https://doi.org/10.1016/j.pharmthera.2008.05.005
- D. Pitocco, T. Spanu, M. Di Leo, R. Vitiello, A. Rizzi et al., Diabetic foot infections: a comprehensive overview. Eur. Rev. Med. Pharmacol. Sci. 23(2 Suppl), 26–37 (2019). https://doi.org/10.26355/eurrev_201904_17471
- J. Apelqvist, Diagnostics and treatment of the diabetic foot. Endocrine 41(3), 384–397 (2012). https://doi.org/10.1007/s12020-012-9619-x
- A.G. Logan, M.J. Irvine, W.J. McIsaac, A. Tisler, P.G. Rossos et al., Effect of home blood pressure telemonitoring with self-care support on uncontrolled systolic hypertension in diabetics. Hypertension 60(1), 51–57 (2012). https://doi.org/10.1161/HYPERTENSIONAHA.111.188409
- J. Apelqvist, J. Castenfors, J. Larsson, A. Stenström, C.D. Agardh, Prognostic value of systolic ankle and toe blood pressure levels in outcome of diabetic foot ulcer. Diabetes Care 12(6), 373–378 (1989). https://doi.org/10.2337/diacare.12.6.373
- S. Ferri, K. Kojima, K. Sode, Review of glucose oxidases and glucose dehydrogenases: a bird’s eye view of glucose sensing enzymes. J. Diabetes Sci. Technol. 5(5), 1068–1076 (2011). https://doi.org/10.1177/193229681100500507
- S.B. Kim, J. Koo, J. Yoon, A. Hourlier-Fargette, B. Lee et al., Soft, skin-interfaced microfluidic systems with integrated enzymatic assays for measuring the concentration of ammonia and ethanol in sweat. Lab Chip 20(1), 84–92 (2020). https://doi.org/10.1039/c9lc01045a
- O. Boutureira, G.J.L. Bernardes, Advances in chemical protein modification. Chem. Rev. 115(5), 2174–2195 (2015). https://doi.org/10.1021/cr500399p
- C.D. Spicer, B.G. Davis, Selective chemical protein modification. Nat. Commun. 5, 4740 (2014). https://doi.org/10.1038/ncomms5740
- X. Cheng, B. Wang, Y. Zhao, H. Hojaiji, S. Lin et al., A mediator-free electroenzymatic sensing methodology to mitigate ionic and electroactive interferents’ effects for reliable wearable metabolite and nutrient monitoring. Adv. Funct. Mater. 30(10), 1908507 (2020). https://doi.org/10.1002/adfm.201908507
- J. Wang, F. Lu, Oxygen-rich oxidase enzyme electrodes for operation in oxygen-free solutions. J. Am. Chem. Soc. 120(5), 1048–1050 (1998). https://doi.org/10.1021/ja972759p
- Y. Horaguchi, S. Saito, K. Kojima, W. Tsugawa, S. Ferri et al., Engineering glucose oxidase to minimize the influence of oxygen on sensor response. Electrochim. Acta 126, 158–161 (2014). https://doi.org/10.1016/j.electacta.2013.09.018
- D.A. Gough, J.Y. Lucisano, P.H.S. Tse, Two-dimensional enzyme electrode sensor for glucose. Anal. Chem. 57(12), 2351–2357 (1985). https://doi.org/10.1021/ac00289a042
- T. Saha, R. Del Caño, K. Mahato, E. De la Paz, C. Chen et al., Wearable electrochemical glucose sensors in diabetes management: a comprehensive review. Chem. Rev. 123(12), 7854–7889 (2023). https://doi.org/10.1021/acs.chemrev.3c00078
- A. Chaubey, B.D. Malhotra, Mediated biosensors. Biosens. Bioelectron. 17(6–7), 441–456 (2002). https://doi.org/10.1016/S0956-5663(01)00313-X
- Y. Lin, M. Bariya, H.Y.Y. Nyein, L. Kivimäki, S. Uusitalo et al., Porous enzymatic membrane for nanotextured glucose sweat sensors with high stability toward reliable noninvasive health monitoring. Adv. Funct. Mater. 29(33), 1902521 (2019). https://doi.org/10.1002/adfm.201902521
- X.T. Zheng, M.W.N. Leoi, Y. Yu, S.C.L. Tan, N. Nadzri et al., Co-encapsulating enzymes and carbon dots in metal–organic frameworks for highly stable and sensitive touch-based sweat sensors. Adv. Funct. Mater. 34(10), 2310121 (2024). https://doi.org/10.1002/adfm.202310121
- D. Zhang, Y. Bai, H. Niu, L. Chen, J. Xiao et al., Enzyme immobilization by inkjet printing on reagentless biosensors for electrochemical phosphate detection. Biosensors 14(4), 168 (2024). https://doi.org/10.3390/bios14040168
- H.-Q. Xia, H. Tang, B. Zhou, Y. Li, X. Zhang et al., Mediator-free electron-transfer on patternable hierarchical meso/macro porous bienzyme interface for highly-sensitive sweat glucose and surface electromyography monitoring. Sens. Actuat. B Chem. 312, 127962 (2020). https://doi.org/10.1016/j.snb.2020.127962
- A. Paul, G. Vyas, P. Paul, D.N. Srivastava, Gold-nanop-encapsulated zif-8 for a mediator-free enzymatic glucose sensor by amperometry. ACS Appl. Nano Mater. 1, 3600–3607 (2018). https://doi.org/10.1021/acsanm.8b00748
- W. Jia, A.J. Bandodkar, G. Valdés-Ramírez, J.R. Windmiller, Z. Yang et al., Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 85(14), 6553–6560 (2013). https://doi.org/10.1021/ac401573r
- A. Wiorek, M. Parrilla, M. Cuartero, G.A. Crespo, Epidermal patch with glucose biosensor: pH and temperature correction toward more accurate sweat analysis during sport practice. Anal. Chem. 92(14), 10153–10161 (2020). https://doi.org/10.1021/acs.analchem.0c02211
- M. Li, L. Wang, R. Liu, J. Li, Q. Zhang et al., A highly integrated sensing paper for wearable electrochemical sweat analysis. Biosens. Bioelectron. 174, 112828 (2021). https://doi.org/10.1016/j.bios.2020.112828
- V. Myndrul, E. Coy, N. Babayevska, V. Zahorodna, V. Balitskyi et al., MXene nanoflakes decorating ZnO tetrapods for enhanced performance of skin-attachable stretchable enzymatic electrochemical glucose sensor. Biosens. Bioelectron. 207, 114141 (2022). https://doi.org/10.1016/j.bios.2022.114141
- W. Gao, S. Emaminejad, H.Y.Y. Nyein, S. Challa, K. Chen et al., Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529(7587), 509–514 (2016). https://doi.org/10.1038/nature16521
- S. Yoon, H. Yoon, M.A. Zahed, C. Park, D. Kim et al., Multifunctional hybrid skin patch for wearable smart healthcare applications. Biosens. Bioelectron. 196, 113685 (2022). https://doi.org/10.1016/j.bios.2021.113685
- W. Suginta, P. Khunkaewla, A. Schulte, Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev. 113(7), 5458–5479 (2013). https://doi.org/10.1021/cr300325r
- L. Wang, J. Lu, Q. Li, L. Li, E. He et al., A core–sheath sensing yarn-based electrochemical fabric system for powerful sweat capture and stable sensing. Adv. Funct. Mater. 32(23), 2200922 (2022). https://doi.org/10.1002/adfm.202200922
- J. Niu, S. Lin, D. Chen, Z. Wang, C. Cao et al., A fully elastic wearable electrochemical sweat detection system of tree-bionic microfluidic structure for real-time monitoring. Small 20(11), 2306769 (2024). https://doi.org/10.1002/smll.202306769
- I. Shitanda, Y. Ozone, Y. Morishita, H. Matsui, N. Loew et al., Air-bubble-insensitive microfluidic lactate biosensor for continuous monitoring of lactate in sweat. ACS Sens. 8(6), 2368–2374 (2023). https://doi.org/10.1021/acssensors.3c00490
- J. Kim, J.R. Sempionatto, S. Imani, M.C. Hartel, A. Barfidokht et al., Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv. Sci. 5(10), 1800880 (2018). https://doi.org/10.1002/advs.201800880
- M. Abu Zahed, M. Sharifuzzaman, H. Yoon, M. Asaduzzaman, D.K. Kim et al., A nanoporous carbon-MXene heterostructured nanocomposite-based epidermal patch for real-time biopotentials and sweat glucose monitoring. Adv. Funct. Mater. 32(49), 2208344 (2022). https://doi.org/10.1002/adfm.202208344
- C. Xu, Y. Song, J.R. Sempionatto, S.A. Solomon, Y. Yu et al., A physicochemical-sensing electronic skin for stress response monitoring. Nat. Electron. 7(2), 168–179 (2024). https://doi.org/10.1038/s41928-023-01116-6
- Z. Wang, J. Shin, J.-H. Park, H. Lee, D.-H. Kim et al., Engineering materials for electrochemical sweat sensing. Adv. Funct. Mater. 31(12), 2008130 (2021). https://doi.org/10.1002/adfm.202008130
- Y. Zhao, B. Wang, H. Hojaiji, Z. Wang, S. Lin et al., A wearable freestanding electrochemical sensing system. Sci. Adv. 6(12), eaaz0007 (2020). https://doi.org/10.1126/sciadv.aaz0007
- X. Huang, C. Yao, S. Huang, S. Zheng, Z. Liu et al., Technological advances of wearable device for continuous monitoring of in vivo glucose. ACS Sens. 9(3), 1065–1088 (2024). https://doi.org/10.1021/acssensors.3c01947
- M. Vestergaard, K. Kerman, E. Tamiya, An overview of label-free electrochemical protein sensors. Sensors 7(12), 3442–3458 (2007). https://doi.org/10.3390/s7123442
- D. Kinnamon, R. Ghanta, K.-C. Lin, S. Muthukumar, S. Prasad, Portable biosensor for monitoring Cortisol in low-volume perspired human sweat. Sci. Rep. 7(1), 13312 (2017). https://doi.org/10.1038/s41598-017-13684-7
- J.S. Nah, S.C. Barman, M. Abu Zahed, M. Sharifuzzaman, H. Yoon et al., A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat Cortisol detection. Sens. Actuat. B Chem. 329, 129206 (2021). https://doi.org/10.1016/j.snb.2020.129206
- S.K. Tuteja, C. Ormsby, S. Neethirajan, Noninvasive label-free detection of Cortisol and lactate using graphene embedded screen-printed electrode. Nano-Micro Lett. 10(3), 41 (2018). https://doi.org/10.1007/s40820-018-0193-5
- M. Sekar, M. Pandiaraj, S. Bhansali, N. Ponpandian, C. Viswanathan, Carbon fiber based electrochemical sensor for sweat Cortisol measurement. Sci. Rep. 9(1), 403 (2019). https://doi.org/10.1038/s41598-018-37243-w
- T. Laochai, J. Yukird, N. Promphet, J. Qin, O. Chailapakul et al., Non-invasive electrochemical immunosensor for sweat Cortisol based on L-cys/AuNPs/MXene modified thread electrode. Biosens. Bioelectron. 203, 114039 (2022). https://doi.org/10.1016/j.bios.2022.114039
- G. Tian, Z. Zhou, M. Li, X. Li, T. Xu et al., Oriented antibody-assembled metal–organic frameworks for persistent wearable sweat Cortisol detection. Anal. Chem. 95(35), 13250–13257 (2023). https://doi.org/10.1021/acs.analchem.3c02392
- S. Demuru, J. Kim, M. El Chazli, S. Bruce, M. Dupertuis et al., Antibody-coated wearable organic electrochemical transistors for Cortisol detection in human sweat. ACS Sens. 7(9), 2721–2731 (2022). https://doi.org/10.1021/acssensors.2c01250
- C. Cheng, X. Li, G. Xu, Y. Lu, S.S. Low et al., Battery-free, wireless, and flexible electrochemical patch for in situ analysis of sweat Cortisol via near field communication. Biosens. Bioelectron. 172, 112782 (2021). https://doi.org/10.1016/j.bios.2020.112782
- J. Aerathupalathu Janardhanan, Y.L. Chen, C.T. Liu, H.S. Tseng, P.I. Wu et al., Sensitive detection of sweat Cortisol using an organic electrochemical transistor featuring nanostructured poly(3,4-ethylenedioxythiophene) derivatives in the channel layer. Anal. Chem. 94(21), 7584–7593 (2022). https://doi.org/10.1021/acs.analchem.2c00497
- H.-J. Jang, T. Lee, J. Song, L. Russell, H. Li et al., Electronic Cortisol detection using an antibody-embedded polymer coupled to a field-effect transistor. ACS Appl. Mater. Interfaces 10(19), 16233–16237 (2018). https://doi.org/10.1021/acsami.7b18855
- B. Jagannath, K.-C. Lin, M. Pali, D. Sankhala, S. Muthukumar et al., A sweat-based wearable enabling technology for real-time monitoring of IL-1β and CRP as potential markers for inflammatory bowel disease. Inflamm. Bowel Dis. 26(10), 1533–1542 (2020). https://doi.org/10.1093/ibd/izaa191
- K. Khachornsakkul, W. Dungchai, N. Pamme, Distance-based all-In-one immunodevice for point-of-care monitoring of cytokine interleukin-6. ACS Sens. 7(8), 2410–2419 (2022). https://doi.org/10.1021/acssensors.2c01122
- R.D. Munje, S. Muthukumar, B. Jagannath, S. Prasad, A new paradigm in sweat based wearable diagnostics biosensors using Room Temperature Ionic Liquids (RTILs). Sci. Rep. 7, 1950 (2017). https://doi.org/10.1038/s41598-017-02133-0
- B. Jagannath, K.-C. Lin, M. Pali, D. Sankhala, S. Muthukumar et al., Temporal profiling of cytokines in passively expressed sweat for detection of infection using wearable device. Bioeng. Transl. Med. 6(3), e10220 (2021). https://doi.org/10.1002/btm2.10220
- G.S. Perera, T. Ahmed, L. Heiss, S. Walia, M. Bhaskaran et al., Rapid and selective biomarker detection with conductometric sensors. Small 17(7), 2005582 (2021). https://doi.org/10.1002/smll.202005582
- T. De Meyer, S. Muyldermans, A. Depicker, Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 32(5), 263–270 (2014). https://doi.org/10.1016/j.tibtech.2014.03.001
- A. Gray, A.R.M. Bradbury, A. Knappik, A. Plückthun, C.A.K. Borrebaeck et al., Animal-free alternatives and the antibody iceberg. Nat. Biotechnol. 38(11), 1234–1239 (2020). https://doi.org/10.1038/s41587-020-0687-9
- J. Zheng, R. Yang, M. Shi, C. Wu, X. Fang et al., Rationally designed molecular beacons for bioanalytical and biomedical applications. Chem. Soc. Rev. 44(10), 3036–3055 (2015). https://doi.org/10.1039/C5CS00020C
- C.P. Rusconi, E. Scardino, J. Layzer, G.A. Pitoc, T.L. Ortel et al., RNA aptamers as reversible antagonists of coagulation factor IXa. Nature 419(6902), 90–94 (2002). https://doi.org/10.1038/nature00963
- S. Song, L. Wang, J. Li, C. Fan, J. Zhao, Aptamer-based biosensors. Trac Trends Anal. Chem. 27(2), 108–117 (2008). https://doi.org/10.1016/j.trac.2007.12.004
- S. Sheibani, L. Capua, S. Kamaei, S.S.A. Akbari, J. Zhang et al., Extended gate field-effect-transistor for sensing Cortisol stress hormone. Commun. Mater. 2(1), 10 (2021). https://doi.org/10.1038/s43246-020-00114-x
- M. Elbadawi, J.J. Ong, T.D. Pollard, S. Gaisford, A.W. Basit, Additive manufacturable materials for electrochemical biosensor electrodes. Adv. Funct. Mater. 31(10), 2006407 (2021). https://doi.org/10.1002/adfm.202006407
- A. Ganguly, K.C. Lin, S. Muthukumar, S. Prasad, Autonomous, real-time monitoring electrochemical aptasensor for circadian tracking of Cortisol hormone in sub-microliter volumes of passively eluted human sweat. ACS Sens. 6(1), 63–72 (2021). https://doi.org/10.1021/acssensors.0c01754
- M. Janghorban, I. Aradanas, K. Malaeb, H. Abuelazm, A. Nittala et al., Redox-concatenated aptamer integrated skin mimicking electrochemical patch for noninvasive detection of Cortisol. ACS Sens. 9(2), 799–809 (2024). https://doi.org/10.1021/acssensors.3c02110
- N.K. Singh, S. Chung, A.-Y. Chang, J. Wang, D.A. Hall, A non-invasive wearable stress patch for real-time Cortisol monitoring using a pseudoknot-assisted aptamer. Biosens. Bioelectron. 227, 115097 (2023). https://doi.org/10.1016/j.bios.2023.115097
- Z. Hao, Z. Wang, Y. Li, Y. Zhu, X. Wang et al., Measurement of cytokine biomarkers using an aptamer-based affinity graphene nanosensor on a flexible substrate toward wearable applications. Nanoscale 10(46), 21681–21688 (2018). https://doi.org/10.1039/C8NR04315A
- Z. Wang, Z. Hao, X. Wang, C. Huang, Q. Lin et al., A flexible and regenerative aptameric graphene–nafion biosensor for cytokine storm biomarker monitoring in undiluted biofluids toward wearable applications. Adv. Funct. Mater. 31(4), 2005958 (2021). https://doi.org/10.1002/adfm.202005958
- C. Huang, D. Li, J. Liu, S. Hou, W. Yang et al., A flexible aptameric graphene field-effect nanosensor capable of automatic liquid collection/filtering for cytokine storm biomarker monitoring in undiluted sweat. Adv. Funct. Mater. 34(9), 2309447 (2024). https://doi.org/10.1002/adfm.202309447
- H. Chu, X. Hu, C.-Y. Lee, A. Zhang, Y. Ye et al., A wearable electrochemical fabric for cytokine monitoring. Biosens. Bioelectron. 232, 115301 (2023). https://doi.org/10.1016/j.bios.2023.115301
- Y. Dong, T.-L. Liu, S. Chen, P. Nithianandam, K. Matar et al., A “two-part” resonance circuit based detachable sweat patch for noninvasive biochemical and biophysical sensing. Adv. Funct. Mater. 33(6), 2210136 (2023). https://doi.org/10.1002/adfm.202210136
- S. Dalirirad, A.J. Steckl, Aptamer-based lateral flow assay for point of care Cortisol detection in sweat. Sens. Actuat. B Chem. 283, 79–86 (2019). https://doi.org/10.1016/j.snb.2018.11.161
- L.S. Liu, F. Wang, Y. Ge, P.K. Lo, Recent developments in aptasensors for diagnostic applications. ACS Appl. Mater. Interfaces 13(8), 9329–9358 (2021). https://doi.org/10.1021/acsami.0c14788
- L. Meng, A.P.F. Turner, W.C. Mak, Soft and flexible material-based affinity sensors. Biotechnol. Adv. 39, 107398 (2020). https://doi.org/10.1016/j.biotechadv.2019.05.004
- S.A. Zaidi, Latest trends in molecular imprinted polymer based drug delivery systems. RSC Adv. 6(91), 88807–88819 (2016). https://doi.org/10.1039/c6ra18911c
- Q. Zhang, D. Jiang, C. Xu, Y. Ge, X. Liu et al., Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat. Sens. Actuat. B Chem. 320, 128325 (2020). https://doi.org/10.1016/j.snb.2020.128325
- H. Zhao, X. Zhang, Y. Qin, Y. Xia, X. Xu et al., An integrated wearable sweat sensing patch for passive continuous analysis of stress biomarkers at rest. Adv. Funct. Mater. 33(9), 2212083 (2023). https://doi.org/10.1002/adfm.202212083
- A. Mani, T.S. Anirudhan, Electrochemical sensing of Cortisol by gold nanop incorporated carboxylated graphene oxide based molecularly imprinted polymer. Chem. Eng. J. 493, 152654 (2024). https://doi.org/10.1016/j.cej.2024.152654
- M.-M. Chen, S.-B. Cheng, K. Ji, J. Gao, Y.-L. Liu et al., Construction of a flexible electrochemiluminescence platform for sweat detection. Chem. Sci. 10(25), 6295–6303 (2019). https://doi.org/10.1039/c9sc01937e
- H. Liu, W. Qin, X. Li, L. Feng, C. Gu et al., Molecularly imprinted electrochemical sensors based on Ti3C2Tx-MXene and graphene composite modifications for ultrasensitive Cortisol detection. Anal. Chem. 95(44), 16079–16088 (2023). https://doi.org/10.1021/acs.analchem.3c01715
- E. Sehit, J. Drzazgowska, D. Buchenau, C. Yesildag, M. Lensen et al., Ultrasensitive nonenzymatic electrochemical glucose sensor based on gold nanops and molecularly imprinted polymers. Biosens. Bioelectron. 165, 112432 (2020). https://doi.org/10.1016/j.bios.2020.112432
- M. Singh, S. Singh, S.P. Singh, S.S. Patel, Recent advancement of carbon nanomaterials engrained molecular imprinted polymer for environmental matrix. Trends Environ. Anal. Chem. 27, e00092 (2020). https://doi.org/10.1016/j.teac.2020.e00092
- L.P.C. Gomez, A. Spangenberg, X.-A. Ton, Y. Fuchs, F. Bokeloh et al., Rapid prototyping of chemical microsensors based on molecularly imprinted polymers synthesized by two-photon stereolithography. Adv. Mater. 28(28), 5931–5937 (2016). https://doi.org/10.1002/adma.201600218
- X. Yang, J. Sun, F. Cui, J. Ji, L. Wang et al., An eco-friendly sensor based on CQD@MIPs for detection of N-acylated homoserine lactones and its 3D printing applications. Talanta 219, 121343 (2020). https://doi.org/10.1016/j.talanta.2020.121343
- J.C. Yang, J. Lee, S.W. Hong, J. Park, Molecularly imprinted quartz crystal microbalance sensors with lithographically patterned frisbee-like pillar arrays for sensitive and selective detection of iprodione. Sens. Actuat. B Chem. 320, 128366 (2020). https://doi.org/10.1016/j.snb.2020.128366
- W. Tang, L. Yin, J.R. Sempionatto, J.-M. Moon, H. Teymourian et al., Touch-based stressless Cortisol sensing. Adv. Mater. 33(18), e2008465 (2021). https://doi.org/10.1002/adma.202008465
- Y. Bai, J. Fu, Z. Qin, Q. Gao, S. Li, Integrated biosensing system of electrochemistry and electrophysiology for Cortisol and skin conductance analysis on smartphone. Sens. Actuat. B Chem. 394, 134368 (2023). https://doi.org/10.1016/j.snb.2023.134368
- G. Dykstra, I. Chapa, Y. Liu, Reagent-free lactate detection using Prussian blue and electropolymerized-molecularly imprinted polymers-based electrochemical biosensors. ACS Appl. Mater. Interfaces 16(49), 66921–66931 (2024). https://doi.org/10.1021/acsami.3c19448
- S. Yeasmin, A. Ullah, B. Wu, X. Zhang, L.-J. Cheng, Enzyme-mimics for sensitive and selective steroid metabolite detection. ACS Appl. Mater. Interfaces (2023). https://doi.org/10.1021/acsami.2c21980
- S. Yeasmin, A. Ullah, B. Wu, X. Zhang, L.-J. Cheng, Hybrid functional polymer-enabled multiplexed chemosensor patch for wearable adrenocortex stress profiling. ACS Appl. Mater. Interfaces 15(43), 50034–50046 (2023). https://doi.org/10.1021/acsami.3c11374
- X. Hu, Y. Chen, X. Wang, K. Jia, H. Zhang et al., Wearable and regenerable electrochemical fabric sensing system based on molecularly imprinted polymers for real-time stress management. Adv. Funct. Mater. 34(14), 2312897 (2024). https://doi.org/10.1002/adfm.202312897
- W.-T. Ting, M.-J. Wang, M.M.R. Howlader, Interleukin-6 electrochemical sensor using poly(o-phenylenediamine)-based molecularly imprinted polymer. Sens. Actuat. B Chem. 404, 135282 (2024). https://doi.org/10.1016/j.snb.2024.135282
- O. Parlak, S.T. Keene, A. Marais, V.F. Curto, A. Salleo, Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive Cortisol sensing. Sci. Adv. 4(7), eaar2904 (2018). https://doi.org/10.1126/sciadv.aar2904
- P. Kanokpaka, L.-Y. Chang, B.-C. Wang, T.-H. Huang, M.-J. Shih et al., Self-powered molecular imprinted polymers-based triboelectric sensor for noninvasive monitoring lactate levels in human sweat. Nano Energy 100, 107464 (2022). https://doi.org/10.1016/j.nanoen.2022.107464
- S. Ramanavicius, A. Ramanavicius, Development of molecularly imprinted polymer based phase boundaries for sensors design (review). Adv. Colloid Interface Sci. 305, 102693 (2022). https://doi.org/10.1016/j.cis.2022.102693
- J.J. BelBruno, Molecularly imprinted polymers. Chem. Rev. 119(1), 94–119 (2019). https://doi.org/10.1021/acs.chemrev.8b00171
- K. Kaewpradub, K. Veenuttranon, H. Jantapaso, P. Mittraparp-Arthorn, I. Jeerapan, A fully-printed wearable bandage-based electrochemical sensor with pH correction for wound infection monitoring. Nano-Micro Lett. 17(1), 71 (2024). https://doi.org/10.1007/s40820
References
A. Chaudhury, C. Duvoor, V.S.R. Dendi, S. Kraleti, A. Chada et al., Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front. Endocrinol. 8, 6 (2017). https://doi.org/10.3389/fendo.2017.00006
H. Lee, Y.J. Hong, S. Baik, T. Hyeon, D.-H. Kim, Enzyme-based glucose sensor: from invasive to wearable device. Adv. Healthc. Mater. 7(8), 1701150 (2018). https://doi.org/10.1002/adhm.201701150
A.I. Vinik, R.E. Maser, B.D. Mitchell, R. Freeman, Diabetic autonomic neuropathy. Diabetes Care 26(5), 1553–1579 (2003). https://doi.org/10.2337/diacare.26.5.1553
Y. Marunaka, Roles of interstitial fluid pH in diabetes mellitus: glycolysis and mitochondrial function. World J. Diabetes 6(1), 125–135 (2015). https://doi.org/10.4239/wjd.v6.i1.125
N.J. Rehrer, Fluid and electrolyte balance in ultra-endurance sport. Sports Med. 31(10), 701–715 (2001). https://doi.org/10.2165/00007256-200131100-00001
K. Lian, H. Feng, S. Liu, K. Wang, Q. Liu et al., Insulin quantification towards early diagnosis of prediabetes/diabetes. Biosens. Bioelectron. 203, 114029 (2022). https://doi.org/10.1016/j.bios.2022.114029
H. Han, Y. Cao, C. Feng, Y. Zheng, K. Dhana et al., Association of a healthy lifestyle with all-cause and cause-specific mortality among individuals with type 2 diabetes: a prospective study in UK biobank. Diabetes Care 45(2), 319–329 (2022). https://doi.org/10.2337/dc21-1512
C.A. Whicher, S. O’Neill, R.I.G. Holt, Diabetes in the UK: 2019. Diabet. Med. 37(2), 242–247 (2020). https://doi.org/10.1111/dme.14225
B.O. Roep, S. Thomaidou, R. van Tienhoven, A. Zaldumbide, Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 17(3), 150–161 (2021). https://doi.org/10.1038/s41574-020-00443-4
K.L. Wolkowicz, E.M. Aiello, E. Vargas, H. Teymourian, F. Tehrani et al., A review of biomarkers in the context of type 1 diabetes: biological sensing for enhanced glucose control. Bioeng. Transl. Med. 6(2), e10201 (2021). https://doi.org/10.1002/btm2.10201
A.L. McCall, Insulin therapy and hypoglycemia. Endocrinol. Metab. Clin. 41(1), 57–87 (2012). https://doi.org/10.1016/j.ecl.2012.03.001
U. Galicia-Garcia, A. Benito-Vicente, S. Jebari, A. Larrea-Sebal, H. Siddiqi et al., Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 21(17), E6275 (2020). https://doi.org/10.3390/ijms21176275
C. Ling, K. Bacos, T. Rönn, Epigenetics of type 2 diabetes mellitus and weight change: a tool for precision medicine? Nat. Rev. Endocrinol. 18(7), 433–448 (2022). https://doi.org/10.1038/s41574-022-00671-w
D. Tomic, J.E. Shaw, D.J. Magliano, The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 18(9), 525–539 (2022). https://doi.org/10.1038/s41574-022-00690-7
J.B. Cole, J.C. Florez, Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 16(7), 377–390 (2020). https://doi.org/10.1038/s41581-020-0278-5
A. Fitero, S.G. Bungau, D.M. Tit, L. Endres, S.A. Khan et al., Comorbidities, associated diseases, and risk assessment in COVID-19: a systematic review. Int. J. Clin. Pract. 2022, 1571826 (2022). https://doi.org/10.1155/2022/1571826
A.W. Stitt, T.M. Curtis, M. Chen, R.J. Medina, G.J. McKay et al., The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 51, 156–186 (2016). https://doi.org/10.1016/j.preteyeres.2015.08.001
M. Kropp, O. Golubnitschaja, A. Mazurakova, L. Koklesova, N. Sargheini et al., Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications-risks and mitigation. EPMA J. 14(1), 21–42 (2023). https://doi.org/10.1007/s13167-023-00314-8
J.L. Gross, M.J. de Azevedo, S.P. Silveiro, L.H. Canani, M.L. Caramori et al., Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28(1), 164–176 (2005). https://doi.org/10.2337/diacare.28.1.164
M.K. Sulaiman, Diabetic nephropathy: recent advances in pathophysiology and challenges in dietary management. Diabetol. Metab. Syndr. 11(1), 7 (2019). https://doi.org/10.1186/s13098-019-0403-4
E.L. Feldman, B.C. Callaghan, R. Pop-Busui, D.W. Zochodne, D.E. Wright et al., Diabetic neuropathy. Nat. Rev. Dis. Primers. 5, 41 (2019). https://doi.org/10.1038/s41572-019-0092-1
W.J. Jeffcoate, K.G. Harding, Diabetic foot ulcers. Lancet 361(9368), 1545–1551 (2003). https://doi.org/10.1016/S0140-6736(03)13169-8
L. Yazdanpanah, M. Nasiri, S. Adarvishi, Literature review on the management of diabetic foot ulcer. World J. Diabetes 6(1), 37–53 (2015). https://doi.org/10.4239/wjd.v6.i1.37
H.E. Resnick, B.V. Howard, Diabetes and cardiovascular disease. Annu. Rev. Med. 53, 245–267 (2002). https://doi.org/10.1146/annurev.med.53.082901.103904
J.R. Petrie, T.J. Guzik, R.M. Touyz, Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can. J. Cardiol. 34(5), 575–584 (2018). https://doi.org/10.1016/j.cjca.2017.12.005
I. Idris, G.A. Thomson, J.C. Sharma, Diabetes mellitus and stroke. Int. J. Clin. Pract. 60(1), 48–56 (2006). https://doi.org/10.1111/j.1368-5031.2006.00682.x
Z. Bloomgarden, R. Chilton, Diabetes and stroke: an important complication. J. Diabetes 13(3), 184–190 (2021). https://doi.org/10.1111/1753-0407.13142
T. Thiruvoipati, C.E. Kielhorn, E.J. Armstrong, Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes. World J. Diabetes 6(7), 961–969 (2015). https://doi.org/10.4239/wjd.v6.i7.961
S.P. Marso, W.R. Hiatt, Peripheral arterial disease in patients with diabetes. J. Am. Coll. Cardiol. 47(5), 921–929 (2006). https://doi.org/10.1016/j.jacc.2005.09.065
L.C. Hofbauer, C.C. Brueck, S.K. Singh, H. Dobnig, Osteoporosis in patients with diabetes mellitus. J. Bone Miner. Res. 22(9), 1317–1328 (2007). https://doi.org/10.1359/jbmr.070510
K. Wongdee, N. Charoenphandhu, Osteoporosis in diabetes mellitus: possible cellular and molecular mechanisms. World J. Diabetes 2(3), 41–48 (2011). https://doi.org/10.4239/wjd.v2.i3.41
C. Sims-Robinson, B. Kim, A. Rosko, E.L. Feldman, How does diabetes accelerate Alzheimer disease pathology? Nat. Rev. Neurol. 6(10), 551–559 (2010). https://doi.org/10.1038/nrneurol.2010.130
S. Pugazhenthi, L. Qin, P.H. Reddy, Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys. Acta BBA Mol. Basis Dis. 1863(5), 1037–1045 (2017). https://doi.org/10.1016/j.bbadis.2016.04.017
M. Kumar, L. Mishra, R. Mohanty, R. Nayak, Diabetes and gum disease: the diabolic Duo. Diabetes Metab. Syndr. Clin. Res. Rev. 8(4), 255–258 (2014). https://doi.org/10.1016/j.dsx.2014.09.022
I.L.C. Chapple, R. Genco, Working group 2 of the joint EFP/AAP workshop, Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP workshop on periodontitis and systemic diseases. J. Clin. Periodontol. 40(s14), S106–S112 (2013). https://doi.org/10.1111/jcpe.12077
S.M. Danna, E. Graham, R.J. Burns, S.S. Deschênes, N. Schmitz, Association between depressive symptoms and cognitive function in persons with diabetes mellitus: a systematic review. PLoS ONE 11(8), e0160809 (2016). https://doi.org/10.1371/journal.pone.0160809
S. Bellary, I. Kyrou, J.E. Brown, C.J. Bailey, Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat. Rev. Endocrinol. 17(9), 534–548 (2021). https://doi.org/10.1038/s41574-021-00512-2
S. Klein, A. Gastaldelli, H. Yki-Järvinen, P.E. Scherer, Why does obesity cause diabetes? Cell Metab. 34(1), 11–20 (2022). https://doi.org/10.1016/j.cmet.2021.12.012
X.R. Pan, G.W. Li, Y.H. Hu, J.X. Wang, W.Y. Yang et al., Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and diabetes study. Diabetes Care 20(4), 537–544 (1997). https://doi.org/10.2337/diacare.20.4.537
J. Tuomilehto, J. Lindström, J.G. Eriksson, T.T. Valle, H. Hämäläinen et al., Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 344(18), 1343–1350 (2001). https://doi.org/10.1056/NEJM200105033441801
W.C. Knowler, E. Barrett-Connor, S.E. Fowler, R.F. Hamman, J.M. Lachin et al., Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346(6), 393–403 (2002). https://doi.org/10.1056/NEJMoa012512
J. Valabhji, E. Barron, D. Bradley, C. Bakhai, J. Fagg et al., Early outcomes from the English national health service diabetes prevention programme. Diabetes Care 43(1), 152–160 (2020). https://doi.org/10.2337/dc19-1425
Y. Wang, C. Wang, K. Li, X. Song, X. Yan et al., Recent advances of nanomedicine-based strategies in diabetes and complications management: diagnostics, monitoring, and therapeutics. J. Control. Release 330, 618–640 (2021). https://doi.org/10.1016/j.jconrel.2021.01.002
J.W. Stevens, K. Khunti, R. Harvey, M. Johnson, L. Preston et al., Preventing the progression to Type 2 diabetes mellitus in adults at high risk: a systematic review and network meta-analysis of lifestyle, pharmacological and surgical interventions. Diabetes Res. Clin. Pract. 107(3), 320–331 (2015). https://doi.org/10.1016/j.diabres.2015.01.027
X. Li, X. Huang, J. Mo, H. Wang, Q. Huang et al., A fully integrated closed-loop system based on mesoporous microneedles-iontophoresis for diabetes treatment. Adv. Sci. 8(16), 2100827 (2021). https://doi.org/10.1002/advs.202100827
Y. Liu, S. Zeng, W. Ji, H. Yao, L. Lin et al., Emerging theranostic nanomaterials in diabetes and its complications. Adv. Sci. 9(3), 2102466 (2022). https://doi.org/10.1002/advs.202102466
S. Szunerits, S. Melinte, A. Barras, Q. Pagneux, A. Voronova et al., The impact of chemical engineering and technological advances on managing diabetes: present and future concepts. Chem. Soc. Rev. 50(3), 2102–2146 (2021). https://doi.org/10.1039/C9CS00886A
J. Song, Y. Zhang, S.Y. Chan, Z. Du, Y. Yan et al., Hydrogel-based flexible materials for diabetes diagnosis, treatment, and management. NPJ Flex. Electron. 5, 26 (2021). https://doi.org/10.1038/s41528-021-00122-y
S.A. Pullano, M. Greco, M.G. Bianco, D. Foti, A. Brunetti et al., Glucose biosensors in clinical practice: principles, limits and perspectives of currently used devices. Theranostics 12(2), 493–511 (2022). https://doi.org/10.7150/thno.64035
W.D. Strain, S.V. Hope, A. Green, P. Kar, J. Valabhji et al., Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty. A national collaborative stakeholder initiative. Diabet. Med. 35(7), 838–845 (2018). https://doi.org/10.1111/dme.13644
A.D. Association, 12. Older adults: Standards of medical care in diabetes-2020. Diabetes Care 43(Suppl 1), S152–S162 (2020). https://doi.org/10.2337/dc20-S012
X. Lu, Q. Xie, X. Pan, R. Zhang, X. Zhang et al., Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 9(1), 262 (2024). https://doi.org/10.1038/s41392-024-01951-9
F.H. Karlsson, V. Tremaroli, I. Nookaew, G. Bergström, C.J. Behre et al., Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498(7452), 99–103 (2013). https://doi.org/10.1038/nature12198
W. Jia, J.C. Chan, T.Y. Wong, E.B. Fisher, Diabetes in China: epidemiology, pathophysiology and multi-omics. Nat. Metab. 7(1), 16–34 (2025). https://doi.org/10.1038/s42255-024-01190-w
J.C.N. Chan, V. Malik, W. Jia, T. Kadowaki, C.S. Yajnik et al., Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 301(20), 2129–2140 (2009). https://doi.org/10.1001/jama.2009.726
T.J. Lyons, A. Basu, Biomarkers in diabetes: hemoglobin A1c, vascular and tissue markers. Transl. Res. 159(4), 303–312 (2012). https://doi.org/10.1016/j.trsl.2012.01.009
C. Guay, R. Regazzi, Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 9(9), 513–521 (2013). https://doi.org/10.1038/nrendo.2013.86
M. Song, H. Bai, P. Zhang, X. Zhou, B. Ying, Promising applications of human-derived saliva biomarker testing in clinical diagnostics. Int. J. Oral Sci. 15, 2 (2023). https://doi.org/10.1038/s41368-022-00209-w
H. de Puig, R.A. Lee, D. Najjar, X. Tan, L.R. Soeknsen et al., Minimally instrumented SHERLOCK (miSHERLOCK) for CRISPR-based point-of-care diagnosis of SARS-CoV-2 and emerging variants. Sci. Adv. 7(32), eabh2944 (2021). https://doi.org/10.1126/sciadv.abh2944
J. Min, J. Tu, C. Xu, H. Lukas, S. Shin et al., Skin-interfaced wearable sweat sensors for precision medicine. Chem. Rev. 123(8), 5049–5138 (2023). https://doi.org/10.1021/acs.chemrev.2c00823
C. Niederberger, A. Vermeersch, F. Davidhi, C.Y. Ewald, G. Havenith et al., Wearable sweat analysis to determine biological age. Trends Biotechnol. 41(9), 1113–1116 (2023). https://doi.org/10.1016/j.tibtech.2023.02.001
Y. Zhu, S. Li, J. Li, N. Falcone, Q. Cui et al., Lab-on-a-contact lens: recent advances and future opportunities in diagnostics and therapeutics. Adv. Mater. 34(24), 2108389 (2022). https://doi.org/10.1002/adma.202108389
K. Kim, H.J. Kim, H. Zhang, W. Park, D. Meyer et al., All-printed stretchable corneal sensor on soft contact lenses for noninvasive and painless ocular electrodiagnosis. Nat. Commun. 12(1), 1544 (2021). https://doi.org/10.1038/s41467-021-21916-8
J. Heikenfeld, A. Jajack, B. Feldman, S.W. Granger, S. Gaitonde et al., Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37(4), 407–419 (2019). https://doi.org/10.1038/s41587-019-0040-3
M. Friedel, I.A.P. Thompson, G. Kasting, R. Polsky, D. Cunningham et al., Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat. Biomed. Eng. 7(12), 1541–1555 (2023). https://doi.org/10.1038/s41551-022-00998-9
H.C. Ates, C. Dincer, Wearable breath analysis. Nat. Rev. Bioeng. 1(2), 80–82 (2023). https://doi.org/10.1038/s44222-022-00011-7
H.C. Ates, P.Q. Nguyen, L. Gonzalez-Macia, E. Morales-Narváez, F. Güder et al., End-to-end design of wearable sensors. Nat. Rev. Mater. 7(11), 887–907 (2022). https://doi.org/10.1038/s41578-022-00460-x
M.C. Brothers, M. DeBrosse, C.C. Grigsby, R.R. Naik, S.M. Hussain et al., Achievements and challenges for real-time sensing of analytes in sweat within wearable platforms. Acc. Chem. Res. 52(2), 297–306 (2019). https://doi.org/10.1021/acs.accounts.8b00555
L. Yang, H. Lv, M. Li, Y. Zhang, J. Liu et al., Multiple polarization effect of shell evolution on hierarchical hollow C@MnO2 composites and their wideband electromagnetic wave absorption properties. Chem. Eng. J. 392, 123666 (2020). https://doi.org/10.1016/j.cej.2019.123666
E.P. Dutkiewicz, H.-Y. Chiu, P.L. Urban, Probing skin for metabolites and topical drugs with hydrogel micropatches. Anal. Chem. 89(5), 2664–2670 (2017). https://doi.org/10.1021/acs.analchem.6b04276
D.P. Elpa, H.-Y. Chiu, S.-P. Wu, P.L. Urban, Skin metabolomics. Trends Endocrinol Metab 32, 66–75 (2021). https://doi.org/10.1016/j.tem.2020.11.009
A.J. Thody, S. Shuster, Control and function of sebaceous glands. Physiol. Rev. 69(2), 383–416 (1989). https://doi.org/10.1152/physrev.1989.69.2.383
J.R. Sempionatto, I. Jeerapan, S. Krishnan, J. Wang, Wearable chemical sensors: emerging systems for on-body analytical chemistry. Anal. Chem. 92(1), 378–396 (2020). https://doi.org/10.1021/acs.analchem.9b04668
Y. Hu, C. Converse, M.C. Lyons, W.H. Hsu, Neural control of sweat secretion: a review. Br. J. Dermatol. 178(6), 1246–1256 (2018). https://doi.org/10.1111/bjd.15808
P.A.J. Kolarsick, M.A. Kolarsick, C. Goodwin, Anatomy and physiology of the skin. J. Dermatol. Nurses’ Assoc. 3(4), 203–213 (2011). https://doi.org/10.1097/jdn.0b013e3182274a98
M. Wang, Y. Yang, J. Min, Y. Song, J. Tu et al., A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6(11), 1225–1235 (2022). https://doi.org/10.1038/s41551-022-00916-z
L.B. Baker, Physiology of sweat gland function: the roles of sweating and sweat composition in human health. Temperature 6(3), 211–259 (2019). https://doi.org/10.1080/23328940.2019.1632145
Y. Zhang, Y. Chen, J. Huang, Y. Liu, J. Peng et al., Skin-interfaced microfluidic devices with one-opening chambers and hydrophobic valves for sweat collection and analysis. Lab Chip 20(15), 2635–2645 (2020). https://doi.org/10.1039/D0LC00400F
J. Choi, D. Kang, S. Han, S.B. Kim, J.A. Rogers, Thin, soft, skin-mounted microfluidic networks with capillary bursting valves for chrono-sampling of sweat. Adv. Healthc. Mater. 6(5), 1601355 (2017). https://doi.org/10.1002/adhm.201601355
H. Shi, Y. Cao, Y. Zeng, Y. Zhou, W. Wen et al., Wearable tesla valve-based sweat collection device for sweat colorimetric analysis. Talanta 240, 123208 (2022). https://doi.org/10.1016/j.talanta.2022.123208
J. Son, G.Y. Bae, S. Lee, G. Lee, S.W. Kim et al., Cactus-spine-inspired sweat-collecting patch for fast and continuous monitoring of sweat. Adv. Mater. 33(40), e2102740 (2021). https://doi.org/10.1002/adma.202102740
L. Wang, T. Xu, X. He, X. Zhang, Flexible, self-healable, adhesive and wearable hydrogel patch for colorimetric sweat detection. J. Mater. Chem. C 9(41), 14938–14945 (2021). https://doi.org/10.1039/d1tc03905a
B. Dai, K. Li, L. Shi, X. Wan, X. Liu et al., Bioinspired Janus textile with conical micropores for human body moisture and thermal management. Adv. Mater. 31(41), 1904113 (2019). https://doi.org/10.1002/adma.201904113
M.A. Yokus, M.A. Daniele, Integrated non-invasive biochemical and biophysical sensing systems for health and performance monitoring: a systems perspective. Biosens. Bioelectron. 184, 113249 (2021). https://doi.org/10.1016/j.bios.2021.113249
A. Hauke, P. Simmers, Y.R. Ojha, B.D. Cameron, R. Ballweg et al., Complete validation of a continuous and blood-correlated sweat biosensing device with integrated sweat stimulation. Lab Chip 18(24), 3750–3759 (2018). https://doi.org/10.1039/C8LC01082J
T. Saha, S. Mukherjee, M.D. Dickey, O.D. Velev, Harvesting and manipulating sweat and interstitial fluid in microfluidic devices. Lab Chip 24(5), 1244–1265 (2024). https://doi.org/10.1039/D3LC00874F
M.J. Patterson, S.D.R. Galloway, M.A. Nimmo, Variations in regional sweat composition in normal human males. Exp. Physiol. 85(6), 869–875 (2000). https://doi.org/10.1017/s0958067000020583
P.J. Derbyshire, H. Barr, F. Davis, S.P.J. Higson, Lactate in human sweat: a critical review of research to the present day. J. Physiol. Sci. 62(6), 429–440 (2012). https://doi.org/10.1007/s12576-012-0213-z
M.J. Buono, N.V.L. Lee, P.W. Miller, The relationship between exercise intensity and the sweat lactate excretion rate. J. Physiol. Sci. 60(2), 103–107 (2010). https://doi.org/10.1007/s12576-009-0073-3
M.C.G.J. Brouwers, J.C. Ham, E. Wisse, S. Misra, S. Landewe et al., Elevated lactate levels in patients with poorly regulated type 1 diabetes and glycogenic hepatopathy: a new feature of Mauriac syndrome. Diabetes Care 38(2), e11–e12 (2015). https://doi.org/10.2337/dc14-2205
K. Van Hoovels, X. Xuan, M. Cuartero, M. Gijssel, M. Swarén et al., Can wearable sweat lactate sensors contribute to sports physiology? ACS Sens. 6(10), 3496–3508 (2021). https://doi.org/10.1021/acssensors.1c01403
J. Moyer, D. Wilson, I. Finkelshtein, B. Wong, R. Potts, Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 14(5), 398–402 (2012). https://doi.org/10.1089/dia.2011.0262
K. Sakaguchi, Y. Hirota, N. Hashimoto, W. Ogawa, T. Hamaguchi et al., Evaluation of a minimally invasive system for measuring glucose area under the curve during oral glucose tolerance tests: usefulness of sweat monitoring for precise measurement. J. Diabetes Sci. Technol. 7(3), 678–688 (2013). https://doi.org/10.1177/193229681300700313
A. Sedighi, M. Montazer, S. Mazinani, Synthesis of wearable and flexible NiP0.1-SnOx/PANI/CuO/cotton towards a non-enzymatic glucose sensor. Biosens. Bioelectron. 135, 192–199 (2019). https://doi.org/10.1016/j.bios.2019.04.010
Y. Pan, R. Yu, Y. Jiang, H. Zhong, Q. Yuan et al., Heterogeneous CuxO nano-skeletons from waste electronics for enhanced glucose detection. Nano-Micro Lett. 16(1), 249 (2024). https://doi.org/10.1007/s40820-024-01467-5
C.J. Harvey, R.F. LeBouf, A.B. Stefaniak, Formulation and stability of a novel artificial human sweat under conditions of storage and use. Toxicol. Vitro 24(6), 1790–1796 (2010). https://doi.org/10.1016/j.tiv.2010.06.016
S. Wang, A. Zhao, G. Li, X. Sun, J. Wang et al., In situ regenerable molecularly imprinted polymer biosensor for electrochemical detection of nonelectroactive branched-chain amino acids in human sweat. Anal. Chem. 96(51), 20287–20295 (2024). https://doi.org/10.1021/acs.analchem.4c05144
G. Cizza, A.H. Marques, F. Eskandari, I.C. Christie, S. Torvik et al., Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: the POWER study. Biol. Psychiatry 64(10), 907–911 (2008). https://doi.org/10.1016/j.biopsych.2008.05.035
C. Huang, W. Yang, H. Wang, S. Huang, S. Gao et al., Flexible/regenerative nanosensor with automatic sweat collection for cytokine storm biomarker detection. ACS Nano 18(32), 21198–21210 (2024). https://doi.org/10.1021/acsnano.4c04456
B. Wang, C. Zhao, Z. Wang, K.-A. Yang, X. Cheng et al., Wearable aptamer-field-effect transistor sensing system for noninvasive Cortisol monitoring. Sci. Adv. 8(1), eabk0967 (2022). https://doi.org/10.1126/sciadv.abk0967
R.M. Torrente-Rodríguez, J. Tu, Y. Yang, J. Min, M. Wang et al., Investigation of Cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2(4), 921–937 (2020). https://doi.org/10.1016/j.matt.2020.01.021
I. Chiodini, G. Adda, A. Scillitani, F. Coletti, V. Morelli et al., Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care 30(1), 83–88 (2007). https://doi.org/10.2337/dc06-1267
J. Ok, S. Park, Y.H. Jung, T.I. Kim, Wearable and implantable Cortisol-sensing electronics for stress monitoring. Adv. Mater. 36(1), e2211595 (2024). https://doi.org/10.1002/adma.202211595
M.S. Rahman, K.S. Hossain, S. Das, S. Kundu, E.O. Adegoke et al., Role of insulin in health and disease: an update. Int. J. Mol. Sci. 22(12), 6403 (2021). https://doi.org/10.3390/ijms22126403
P.J. Hantzidiamantis, S.L. Lappin, Physiology, glucose. (2019).
M. Tesauro, F.A. Mazzotta, Chapter 3—Pathophysiology of diabetes, in Transplantation, bioengineering, and regeneration of the endocrine pancreas. ed. by G. Orlando, L. Piemonti, C. Ricordi, R.J. Stratta, R.W. Gruessner (Elsevier, Hoboken, 2020), pp.37–47. https://doi.org/10.1016/B978-0-12-814833-4.00003-4
S.E. Kahn, M.E. Cooper, S. Del Prato, Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383(9922), 1068–1083 (2014). https://doi.org/10.1016/S0140-6736(13)62154-6
R.J. Wright, B.M. Frier, Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab. Res. Rev. 24(5), 353–363 (2008). https://doi.org/10.1002/dmrr.865
R.M. Sapolsky, Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress 1(1), 1–19 (1996). https://doi.org/10.3109/10253899609001092
S. Vaddiraju, D.J. Burgess, I. Tomazos, F.C. Jain, F. Papadimitrakopoulos, Technologies for continuous glucose monitoring: current problems and future promises. J. Diabetes Sci. Technol. 4(6), 1540–1562 (2010). https://doi.org/10.1177/193229681000400632
S. Emaminejad, W. Gao, E. Wu, Z.A. Davies, H. Yin Nyein et al., Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. U.S.A. 114(18), 4625–4630 (2017). https://doi.org/10.1073/pnas.1701740114
J.R. Sempionatto, J.-M. Moon, J. Wang, Touch-based fingertip blood-free reliable glucose monitoring: personalized data processing for predicting blood glucose concentrations. ACS Sens. 6(5), 1875–1883 (2021). https://doi.org/10.1021/acssensors.1c00139
E.V. Karpova, E.V. Shcherbacheva, A.A. Galushin, D.V. Vokhmyanina, E.E. Karyakina et al., Noninvasive diabetes monitoring through continuous analysis of sweat using flow-through glucose biosensor. Anal. Chem. 91(6), 3778–3783 (2019). https://doi.org/10.1021/acs.analchem.8b05928
H.Y.Y. Nyein, M. Bariya, L. Kivimäki, S. Uusitalo, T.S. Liaw et al., Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 5(8), eaaw9906 (2019). https://doi.org/10.1126/sciadv.aaw9906
L. Klous, C.J. de Ruiter, S. Scherrer, N. Gerrett, H.A.M. Daanen, The (in)dependency of blood and sweat sodium, chloride, potassium, ammonia, lactate and glucose concentrations during submaximal exercise. Eur. J. Appl. Physiol. 121(3), 803–816 (2021). https://doi.org/10.1007/s00421-020-04562-8
N. Davis, J. Heikenfeld, C. Milla, A. Javey, The challenges and promise of sweat sensing. Nat. Biotechnol. 42(6), 860–871 (2024). https://doi.org/10.1038/s41587-023-02059-1
J.D. Rabinowitz, S. Enerbäck, Lactate: the ugly duckling of energy metabolism. Nat. Metab. 2(7), 566–571 (2020). https://doi.org/10.1038/s42255-020-0243-4
I. San-Millán, G.A. Brooks, Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 38(2), 119–133 (2017). https://doi.org/10.1093/carcin/bgw127
M. Adeva-Andany, M. López-Ojén, R. Funcasta-Calderón, E. Ameneiros-Rodríguez, C. Donapetry-García et al., Comprehensive review on lactate metabolism in human health. Mitochondrion 17, 76–100 (2014). https://doi.org/10.1016/j.mito.2014.05.007
V. Qvisth, E. Hagström-Toft, E. Moberg, S. Sjöberg, J. Bolinder, Lactate release from adipose tissue and skeletal muscle in vivo: defective insulin regulation in insulin-resistant obese women. Am. J. Physiol. Endocrinol. Metab. 292(3), E709–E714 (2007). https://doi.org/10.1152/ajpendo.00104.2006
S.O. Crawford, R.C. Hoogeveen, F.L. Brancati, B.C. Astor, C.M. Ballantyne et al., Association of blood lactate with type 2 diabetes: the Atherosclerosis Risk in Communities Carotid MRI Study. Int. J. Epidemiol. 39(6), 1647–1655 (2010). https://doi.org/10.1093/ije/dyq126
L. Metz, P. Sirvent, G. Py, J.-F. Brun, C. Fédou et al., Relationship between blood lactate concentration and substrate utilization during exercise in type 2 diabetic postmenopausal women. Metabolism 54(8), 1102–1107 (2005). https://doi.org/10.1016/j.metabol.2005.03.015
F. Berhane, A. Fite, N. Daboul, W. Al-Janabi, Z. Msallaty et al., Plasma lactate levels increase during hyperinsulinemic euglycemic clamp and oral glucose tolerance test. J. Diabetes Res. 2015, 102054 (2015). https://doi.org/10.1155/2015/102054
E.V. Karpova, A.I. Laptev, E.A. Andreev, E.E. Karyakina, A.A. Karyakin, Relationship between sweat and blood lactate levels during exhaustive physical exercise. ChemElectroChem 7(1), 191–194 (2020). https://doi.org/10.1002/celc.201901703
A. Márquez, J. Aymerich, M. Dei, R. Rodríguez-Rodríguez, M. Vázquez-Carrera et al., Reconfigurable multiplexed point of care system for monitoring type 1 diabetes patients. Biosens. Bioelectron. 136, 38–46 (2019). https://doi.org/10.1016/j.bios.2019.04.015
N.A. Taylor, C.A. Machado-Moreira, Regional variations in transepidermal water loss, eccrine sweat gland density, sweat secretion rates and electrolyte composition in resting and exercising humans. Extreme Physiol. Med. 2(1), 4 (2013). https://doi.org/10.1186/2046-7648-2-4
C.B. Newgard, J. An, J.R. Bain, M.J. Muehlbauer, R.D. Stevens et al., A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9(4), 311–326 (2009). https://doi.org/10.1016/j.cmet.2009.02.002
X. Chen, W. Yang, Branched-chain amino acids and the association with type 2 diabetes. J. Diabetes Investig. 6(4), 369–370 (2015). https://doi.org/10.1111/jdi.12345
T.J. Wang, M.G. Larson, R.S. Vasan, S. Cheng, E.P. Rhee et al., Metabolite profiles and the risk of developing diabetes. Nat. Med. 17(4), 448–453 (2011). https://doi.org/10.1038/nm.2307
M. Jaromy, J.D. Miller, Potential clinical applications for continuous ketone monitoring in the hospitalized patient with diabetes. Curr. Diab. Rep. 22(10), 501–510 (2022). https://doi.org/10.1007/s11892-022-01489-6
V.A. Fonseca, M.A. Haggar, Achieving glycaemic targets with basal insulin in T2DM by individualizing treatment. Nat. Rev. Endocrinol. 10(5), 276–281 (2014). https://doi.org/10.1038/nrendo.2014.17
F. Vanweert, M. de Ligt, J. Hoeks, M.K.C. Hesselink, P. Schrauwen et al., Elevated plasma branched-chain amino acid levels correlate with type 2 diabetes-related metabolic disturbances. J. Clin. Endocrinol. Metab. 106(4), e1827–e1836 (2021). https://doi.org/10.1210/clinem/dgaa751
H. Nakamura, H. Jinzu, K. Nagao, Y. Noguchi, N. Shimba et al., Plasma amino acid profiles are associated with insulin, C-peptide and adiponectin levels in type 2 diabetic patients. Nutr. Diabetes 4(9), e133 (2014). https://doi.org/10.1038/nutd.2014.32
Y. Zheng, Y. Li, Q. Qi, A. Hruby, J.E. Manson et al., Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int. J. Epidemiol. 45(5), 1482–1492 (2016). https://doi.org/10.1093/ije/dyw143
A. Ray, Cytokines and their role in health and disease: a brief overview. MOJ Immunol. 4(2), 00121 (2016). https://doi.org/10.15406/moji.2016.04.00121
S. Tsalamandris, A.S. Antonopoulos, E. Oikonomou, G.-A. Papamikroulis, G. Vogiatzi et al., The role of inflammation in diabetes: current concepts and future perspectives. Eur. Cardiol. 14(1), 50–59 (2019). https://doi.org/10.15420/ecr.2018.33.1
V. Wieser, A.R. Moschen, H. Tilg, Inflammation, cytokines and insulin resistance: a clinical perspective. Arch. Immunol. Ther. Exp. 61(2), 119–125 (2013). https://doi.org/10.1007/s00005-012-0210-1
A. Rabinovitch, W.L. Suarez-Pinzon, Role of cytokines in the pathogenesis of autoimmune diabetes mellitus. Rev. Endocr. Metab. Dis. 4, 291–299 (2003). https://doi.org/10.1023/A:1025160614313
Q. Li, B. Xu, S.A. Michie, K.H. Rubins, R.D. Schreriber et al., Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc. Natl. Acad. Sci. U.S.A. 105(34), 12439–12444 (2008). https://doi.org/10.1073/pnas.0806439105
H. Thomas, J. Trapani, T. Kay, The role of perforin and granzymes in diabetes. Cell Death Differ. 17, 577–585 (2010). https://doi.org/10.1038/cdd.2009.165
P. Marques-Vidal, F. Bastardot, R. von Känel, F. Paccaud, M. Preisig et al., Association between circulating cytokine levels, diabetes and insulin resistance in a population-based sample (CoLaus study). Clin. Endocrinol. 78(2), 232–241 (2013). https://doi.org/10.1111/j.1365-2265.2012.04384.x
I. Hameed, S.R. Masoodi, S.A. Mir, M. Nabi, K. Ghazanfar et al., Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J. Diabetes 6(4), 598–612 (2015). https://doi.org/10.4239/wjd.v6.i4.598
A. Marques-Deak, G. Cizza, F. Eskandari, S. Torvik, I.C. Christie et al., Measurement of cytokines in sweat patches and plasma in healthy women: validation in a controlled study. J. Immunol. Methods 315(1–2), 99–109 (2006). https://doi.org/10.1016/j.jim.2006.07.011
V. Syngle, A. Syngle, N. Garg, P. Krishan, I. Verma, Predictors of autonomic neuropathy in rheumatoid arthritis. Auton. Neurosci. 201, 54–59 (2016). https://doi.org/10.1016/j.autneu.2016.07.008
T. Kuo, A. McQueen, T.-C. Chen, J.-C. Wang, Regulation of glucose homeostasis by glucocorticoids. Glucocorticoid Signaling (Springer, New York, 2015), pp.99–126. https://doi.org/10.1007/978-1-4939-2895-8_5
P.H. Black, The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med. Hypotheses 67(4), 879–891 (2006). https://doi.org/10.1016/j.mehy.2006.04.008
P. Anagnostis, V.G. Athyros, K. Tziomalos, A. Karagiannis, D.P. Mikhailidis, The pathogenetic role of Cortisol in the metabolic syndrome: a hypothesis. J. Clin. Endocrinol. Metab. 94(8), 2692–2701 (2009). https://doi.org/10.1210/jc.2009-0370
S. Khani, J.A. Tayek, Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome. Clin. Sci. 101(6), 739–747 (2001). https://doi.org/10.1042/cs1010739
P.R. Bratusch-Marrain, Insulin-counteracting hormones: their impact on glucose metabolism. Diabetologia 24(2), 74–79 (1983). https://doi.org/10.1007/BF00297384
R.A. Hackett, A. Steptoe, Type 2 diabetes mellitus and psychological stress—a modifiable risk factor. Nat. Rev. Endocrinol. 13(9), 547–560 (2017). https://doi.org/10.1038/nrendo.2017.64
P. Pearlmutter, G. DeRose, C. Samson, N. Linehan, Y. Cen et al., Sweat and saliva Cortisol response to stress and nutrition factors. Sci. Rep. 10(1), 19050 (2020). https://doi.org/10.1038/s41598-020-75871-3
E. Russell, G. Koren, M. Rieder, S.H.M. Van Uum, The detection of Cortisol in human sweat: implications for measurement of Cortisol in hair. Ther. Drug Monit. 36(1), 30–34 (2014). https://doi.org/10.1097/FTD.0b013e31829daa0a
D. Shabeeb, M. Najafi, G. Hasanzadeh, M.R. Hadian, A.E. Musa et al., Electrophysiological measurements of diabetic peripheral neuropathy: a systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 12(4), 591–600 (2018). https://doi.org/10.1016/j.dsx.2018.03.026
P. Novak, Electrochemical skin conductance: a systematic review. Clin. Auton. Res. 29(1), 17–29 (2019). https://doi.org/10.1007/s10286-017-0467-x
C.M. Casellini, H.K. Parson, M.S. Richardson, M.L. Nevoret, A.I. Vinik, Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol. Ther. 15(11), 948–953 (2013). https://doi.org/10.1089/dia.2013.0129
J.D. England, G.S. Gronseth, G. Franklin, R.G. Miller, A.K. Asbury et al., Distal symmetric polyneuropathy: a definition for clinical research: report of the American academy of neurology, the American association of electrodiagnostic medicine, and the American academy of physical medicine and rehabilitation. Neurology 64(2), 199–207 (2005). https://doi.org/10.1212/01.WNL.0000149522.32823.EA
A. Hovaguimian, C.H. Gibbons, Diagnosis and treatment of pain in small-fiber neuropathy. Curr. Pain Headache Rep. 15(3), 193–200 (2011). https://doi.org/10.1007/s11916-011-0181-7
B.I. Freedman, S.C. Smith, B.M. Bagwell, J. Xu, D.W. Bowden et al., Electrochemical skin conductance in diabetic kidney disease. Am. J. Nephrol. 41(6), 438–447 (2015). https://doi.org/10.1159/000437342
S. Kim, J. Cho, B. Ku, M. Jun, G. Kim et al., Variability of electrochemical skin conductance for screening diabetes mellitus. Biomed. Eng. Lett. 9(2), 267–274 (2019). https://doi.org/10.1007/s13534-019-00111-1
T. He, C. Wang, A. Zuo, P. Liu, R. Zhao et al., Electrochemical skin conductance may be used to screen for diabetic cardiac autonomic neuropathy in a Chinese population with diabetes. J. Diabetes Res. 2017(1), 8289740 (2017). https://doi.org/10.1155/2017/8289740
Y.-R. Lai, C.-C. Huang, B.-C. Cheng, N.-W. Tsai, W.-C. Chiu et al., Feasibility of combining heart rate variability and electrochemical skin conductance as screening and severity evaluation of cardiovascular autonomic neuropathy in type 2 diabetes. J. Diabetes Investig. 12(9), 1671–1679 (2021). https://doi.org/10.1111/jdi.13518
Y.-R. Lai, C.-C. Huang, W.-C. Chiu, B.-C. Cheng, T.-Y. Lin et al., Predictive value of heart rate variability and electrochemical skin conductance measurements for cardiovascular autonomic neuropathy persistence in type 2 diabetes and prediabetes: a 3-year follow-up study. Neurophysiol. Clin. 54(3), 102946 (2024). https://doi.org/10.1016/j.neucli.2024.102946
K. Mohammedi, M. Woodward, Y. Hirakawa, S. Zoungas, B. Williams et al., Microvascular and macrovascular disease and risk for major peripheral arterial disease in patients with type 2 diabetes. Diabetes Care 39(10), 1796–1803 (2016). https://doi.org/10.2337/dc16-0588
M.J. Fowler, Microvascular and macrovascular complications of diabetes. Clin. Diabetes 29(3), 116–122 (2011). https://doi.org/10.2337/diaclin.29.3.116
T. Shinjo, F. Nishimura, The bidirectional association between diabetes and periodontitis, from basic to clinical. Jpn. Dent. Sci. Rev. 60, 15–21 (2024). https://doi.org/10.1016/j.jdsr.2023.12.002
H. Kitaoka, M. Majima, A. Kitazawa, S. Sakane, K. Takeda et al., Impaired regulation of skin temperature in patients with diabetes mellitus evaluated by the cold exposure test. Bull. Osaka Med. Coll. 35(1–2), 99–105 (1989)
J. Petrofsky, L. Berk, F. Alshammari, H. Lee, A. Hamdan et al., The effect of moist air on skin blood flow and temperature in subjects with and without diabetes. Diabetes Technol. Ther. 14(2), 105–116 (2012). https://doi.org/10.1089/dia.2011.0128
S. Bagavathiappan, J. Philip, T. Jayakumar, B. Raj, P.N. Rao et al., Correlation between plantar foot temperature and diabetic neuropathy: a case study by using an infrared thermal imaging technique. J. Diabetes Sci. Technol. 4(6), 1386–1392 (2010). https://doi.org/10.1177/193229681000400613
V.J. Houghton, V.M. Bower, D.C. Chant, Is an increase in skin temperature predictive of neuropathic foot ulceration in people with diabetes? A systematic review and meta-analysis. J. Foot Ankle Res. 6(1), 31 (2013). https://doi.org/10.1186/1757-1146-6-31
J.J. van Netten, M. Prijs, J.G. van Baal, C. Liu, F. van der Heijden et al., Diagnostic values for skin temperature assessment to detect diabetes-related foot complications. Diabetes Technol. Ther. 16(11), 714–721 (2014). https://doi.org/10.1089/dia.2014.0052
A. Berbudi, N. Rahmadika, A.I. Tjahjadi, R. Ruslami, Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 16(5), 442–449 (2020). https://doi.org/10.2174/1573399815666191024085838
L. Korbel, J.D. Spencer, Diabetes mellitus and infection: an evaluation of hospital utilization and management costs in the United States. J. Diabetes Complicat. 29(2), 192–195 (2015). https://doi.org/10.1016/j.jdiacomp.2014.11.005
T.V. Rohm, D.T. Meier, J.M. Olefsky, M.Y. Donath, Inflammation in obesity, diabetes, and related disorders. Immunity 55(1), 31–55 (2022). https://doi.org/10.1016/j.immuni.2021.12.013
B.J. Sparks-DeFriese, Chapter 29—Vascular ulcers, in Physical rehabilitation. ed. by M.H. Cameron, L.G. Monroe (W.B. Saunders, Saint Louis, 2007), pp.777–802. https://doi.org/10.1016/B978-072160361-2.50032-6
F. Andrasik, C. Rime, Chapter 130—Biofeedback, in Pain management (second edition). ed. by S.D. Waldman (W.B. Saunders, Philadelphia, 2011), pp.954–962. https://doi.org/10.1016/B978-1-4377-0721-2.00130-6
S. Singaram, K. Ramakrishnan, J. Selvam, M. Senthil, V. Narayanamurthy, Sweat gland morphology and physiology in diabetes, neuropathy, and nephropathy: a review. Arch. Physiol. Biochem. 130(4), 437–451 (2024). https://doi.org/10.1080/13813455.2022.2114499
T. Schlereth, M. Dieterich, F. Birklein, Hyperhidrosis: causes and treatment of enhanced sweating. Dtsch. Arztebl. Int. 106(3), 32–37 (2009). https://doi.org/10.3238/arztebl.2009.0032
B.M.W. Illigens, C.H. Gibbons, Sweat testing to evaluate autonomic function. Clin. Auton. Res. 19(2), 79–87 (2009). https://doi.org/10.1007/s10286-008-0506-8
V. Provitera, M. Nolano, G. Caporaso, A. Stancanelli, L. Santoro et al., Evaluation of sudomotor function in diabetes using the dynamic sweat test. Neurology 74(1), 50–56 (2010). https://doi.org/10.1212/WNL.0b013e3181c7da4b
W.R. Kennedy, M. Sakuta, D. Sutherland, F.C. Goetz, Quantitation of the sweating deficiency in diabetes mellitus. Ann. Neurol. 15(5), 482–488 (1984). https://doi.org/10.1002/ana.410150514
M. Asahina, Y. Yamanaka, Y. Akaogi, S. Kuwabara, Y. Koyama et al., Measurements of sweat response and skin vasomotor reflex for assessment of autonomic dysfunction in patients with diabetes. J. Diabetes Complicat. 22(4), 278–283 (2008). https://doi.org/10.1016/j.jdiacomp.2007.03.009
S. Stern, S. Sclarowsky, The ECG in diabetes mellitus. Circulation 120(16), 1633–1636 (2009). https://doi.org/10.1161/circulationaha.109.897496
J.J. McMurray, H. Uno, P. Jarolim, A.S. Desai, D. de Zeeuw et al., Predictors of fatal and nonfatal cardiovascular events in patients with type 2 diabetes mellitus, chronic kidney disease, and Anemia: an analysis of the Trial to Reduce cardiovascular Events with Aranesp (darbepoetin-Alfa) Therapy (TREAT). Am. Heart J. 162(4), 748-755.e3 (2011). https://doi.org/10.1016/j.ahj.2011.07.016
B.S. Rana, M.M. Band, S. Ogston, A.D. Morris, S.D. Pringle et al., Relation of QT interval dispersion to the number of different cardiac abnormalities in diabetes mellitus. Am. J. Cardiol. 90(5), 483–487 (2002). https://doi.org/10.1016/s0002-9149(02)02518-3
V.S. Gokhale, M.P. Jeyaseelan, Detailed ECG analysis in type 2 diabetes mellitus: a predictor of multitude of complications. Int. J. Res. Med. Sci. 8(3), 1030 (2020). https://doi.org/10.18203/2320-6012.ijrms20200775
B.M. Leon, Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 6(13), 1246 (2015). https://doi.org/10.4239/wjd.v6.i13.1246
J.R. Sowers, M. Epstein, E.D. Frohlich, Diabetes, hypertension, and cardiovascular disease. Hypertension 37(4), 1053–1059 (2001). https://doi.org/10.1161/01.hyp.37.4.1053
P. Lopez-Jaramillo, J. Lopez-Lopez, C. Lopez-Lopez, M.I. Rodriguez-Alvarez, The goal of blood pressure in the hypertensive patient with diabetes is defined: now the challenge is go from recommendations to practice. Diabetol. Metab. Syndr. 6(1), 31 (2014). https://doi.org/10.1186/1758-5996-6-31
P. Passarella, T.A. Kiseleva, F.V. Valeeva, A.R. Gosmanov, Hypertension management in diabetes: 2018 update. Diabetes Spectr. 31(3), 218–224 (2018). https://doi.org/10.2337/ds17-0085
G.L. Bakris, The importance of blood pressure control in the patient with diabetes. Am. J. Med. 116(5), 30–38 (2004). https://doi.org/10.1016/j.amjmed.2003.10.018
C.A. Emdin, K. Rahimi, B. Neal, T. Callender, V. Perkovic et al., Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 313(6), 603–615 (2015). https://doi.org/10.1001/jama.2014.18574
M.A. Hill, Y. Yang, L. Zhang, Z. Sun, G. Jia et al., Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 119, 154766 (2021). https://doi.org/10.1016/j.metabol.2021.154766
G. Jia, J.R. Sowers, Hypertension in diabetes: an update of basic mechanisms and clinical disease. Hypertension 78(5), 1197–1205 (2021). https://doi.org/10.1161/HYPERTENSIONAHA.121.17981
A.A. da Silva, J.M. do Carmo, X. Li, Z. Wang, A.J. Mouton et al., Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Can. J. Cardiol. 36(5), 671–682 (2020). https://doi.org/10.1016/j.cjca.2020.02.066
E. Ferrannini, W.C. Cushman, Diabetes and hypertension: the bad companions. Lancet 380(9841), 601–610 (2012). https://doi.org/10.1016/S0140-6736(12)60987-8
K. Pickwell, V. Siersma, M. Kars, J. Apelqvist, K. Bakker et al., Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care 38(5), 852–857 (2015). https://doi.org/10.2337/dc14-1598
J.L. Edwards, A.M. Vincent, H.T. Cheng, E.L. Feldman, Diabetic neuropathy: mechanisms to management. Pharmacol. Ther. 120, 1–34 (2008). https://doi.org/10.1016/j.pharmthera.2008.05.005
D. Pitocco, T. Spanu, M. Di Leo, R. Vitiello, A. Rizzi et al., Diabetic foot infections: a comprehensive overview. Eur. Rev. Med. Pharmacol. Sci. 23(2 Suppl), 26–37 (2019). https://doi.org/10.26355/eurrev_201904_17471
J. Apelqvist, Diagnostics and treatment of the diabetic foot. Endocrine 41(3), 384–397 (2012). https://doi.org/10.1007/s12020-012-9619-x
A.G. Logan, M.J. Irvine, W.J. McIsaac, A. Tisler, P.G. Rossos et al., Effect of home blood pressure telemonitoring with self-care support on uncontrolled systolic hypertension in diabetics. Hypertension 60(1), 51–57 (2012). https://doi.org/10.1161/HYPERTENSIONAHA.111.188409
J. Apelqvist, J. Castenfors, J. Larsson, A. Stenström, C.D. Agardh, Prognostic value of systolic ankle and toe blood pressure levels in outcome of diabetic foot ulcer. Diabetes Care 12(6), 373–378 (1989). https://doi.org/10.2337/diacare.12.6.373
S. Ferri, K. Kojima, K. Sode, Review of glucose oxidases and glucose dehydrogenases: a bird’s eye view of glucose sensing enzymes. J. Diabetes Sci. Technol. 5(5), 1068–1076 (2011). https://doi.org/10.1177/193229681100500507
S.B. Kim, J. Koo, J. Yoon, A. Hourlier-Fargette, B. Lee et al., Soft, skin-interfaced microfluidic systems with integrated enzymatic assays for measuring the concentration of ammonia and ethanol in sweat. Lab Chip 20(1), 84–92 (2020). https://doi.org/10.1039/c9lc01045a
O. Boutureira, G.J.L. Bernardes, Advances in chemical protein modification. Chem. Rev. 115(5), 2174–2195 (2015). https://doi.org/10.1021/cr500399p
C.D. Spicer, B.G. Davis, Selective chemical protein modification. Nat. Commun. 5, 4740 (2014). https://doi.org/10.1038/ncomms5740
X. Cheng, B. Wang, Y. Zhao, H. Hojaiji, S. Lin et al., A mediator-free electroenzymatic sensing methodology to mitigate ionic and electroactive interferents’ effects for reliable wearable metabolite and nutrient monitoring. Adv. Funct. Mater. 30(10), 1908507 (2020). https://doi.org/10.1002/adfm.201908507
J. Wang, F. Lu, Oxygen-rich oxidase enzyme electrodes for operation in oxygen-free solutions. J. Am. Chem. Soc. 120(5), 1048–1050 (1998). https://doi.org/10.1021/ja972759p
Y. Horaguchi, S. Saito, K. Kojima, W. Tsugawa, S. Ferri et al., Engineering glucose oxidase to minimize the influence of oxygen on sensor response. Electrochim. Acta 126, 158–161 (2014). https://doi.org/10.1016/j.electacta.2013.09.018
D.A. Gough, J.Y. Lucisano, P.H.S. Tse, Two-dimensional enzyme electrode sensor for glucose. Anal. Chem. 57(12), 2351–2357 (1985). https://doi.org/10.1021/ac00289a042
T. Saha, R. Del Caño, K. Mahato, E. De la Paz, C. Chen et al., Wearable electrochemical glucose sensors in diabetes management: a comprehensive review. Chem. Rev. 123(12), 7854–7889 (2023). https://doi.org/10.1021/acs.chemrev.3c00078
A. Chaubey, B.D. Malhotra, Mediated biosensors. Biosens. Bioelectron. 17(6–7), 441–456 (2002). https://doi.org/10.1016/S0956-5663(01)00313-X
Y. Lin, M. Bariya, H.Y.Y. Nyein, L. Kivimäki, S. Uusitalo et al., Porous enzymatic membrane for nanotextured glucose sweat sensors with high stability toward reliable noninvasive health monitoring. Adv. Funct. Mater. 29(33), 1902521 (2019). https://doi.org/10.1002/adfm.201902521
X.T. Zheng, M.W.N. Leoi, Y. Yu, S.C.L. Tan, N. Nadzri et al., Co-encapsulating enzymes and carbon dots in metal–organic frameworks for highly stable and sensitive touch-based sweat sensors. Adv. Funct. Mater. 34(10), 2310121 (2024). https://doi.org/10.1002/adfm.202310121
D. Zhang, Y. Bai, H. Niu, L. Chen, J. Xiao et al., Enzyme immobilization by inkjet printing on reagentless biosensors for electrochemical phosphate detection. Biosensors 14(4), 168 (2024). https://doi.org/10.3390/bios14040168
H.-Q. Xia, H. Tang, B. Zhou, Y. Li, X. Zhang et al., Mediator-free electron-transfer on patternable hierarchical meso/macro porous bienzyme interface for highly-sensitive sweat glucose and surface electromyography monitoring. Sens. Actuat. B Chem. 312, 127962 (2020). https://doi.org/10.1016/j.snb.2020.127962
A. Paul, G. Vyas, P. Paul, D.N. Srivastava, Gold-nanop-encapsulated zif-8 for a mediator-free enzymatic glucose sensor by amperometry. ACS Appl. Nano Mater. 1, 3600–3607 (2018). https://doi.org/10.1021/acsanm.8b00748
W. Jia, A.J. Bandodkar, G. Valdés-Ramírez, J.R. Windmiller, Z. Yang et al., Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 85(14), 6553–6560 (2013). https://doi.org/10.1021/ac401573r
A. Wiorek, M. Parrilla, M. Cuartero, G.A. Crespo, Epidermal patch with glucose biosensor: pH and temperature correction toward more accurate sweat analysis during sport practice. Anal. Chem. 92(14), 10153–10161 (2020). https://doi.org/10.1021/acs.analchem.0c02211
M. Li, L. Wang, R. Liu, J. Li, Q. Zhang et al., A highly integrated sensing paper for wearable electrochemical sweat analysis. Biosens. Bioelectron. 174, 112828 (2021). https://doi.org/10.1016/j.bios.2020.112828
V. Myndrul, E. Coy, N. Babayevska, V. Zahorodna, V. Balitskyi et al., MXene nanoflakes decorating ZnO tetrapods for enhanced performance of skin-attachable stretchable enzymatic electrochemical glucose sensor. Biosens. Bioelectron. 207, 114141 (2022). https://doi.org/10.1016/j.bios.2022.114141
W. Gao, S. Emaminejad, H.Y.Y. Nyein, S. Challa, K. Chen et al., Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529(7587), 509–514 (2016). https://doi.org/10.1038/nature16521
S. Yoon, H. Yoon, M.A. Zahed, C. Park, D. Kim et al., Multifunctional hybrid skin patch for wearable smart healthcare applications. Biosens. Bioelectron. 196, 113685 (2022). https://doi.org/10.1016/j.bios.2021.113685
W. Suginta, P. Khunkaewla, A. Schulte, Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev. 113(7), 5458–5479 (2013). https://doi.org/10.1021/cr300325r
L. Wang, J. Lu, Q. Li, L. Li, E. He et al., A core–sheath sensing yarn-based electrochemical fabric system for powerful sweat capture and stable sensing. Adv. Funct. Mater. 32(23), 2200922 (2022). https://doi.org/10.1002/adfm.202200922
J. Niu, S. Lin, D. Chen, Z. Wang, C. Cao et al., A fully elastic wearable electrochemical sweat detection system of tree-bionic microfluidic structure for real-time monitoring. Small 20(11), 2306769 (2024). https://doi.org/10.1002/smll.202306769
I. Shitanda, Y. Ozone, Y. Morishita, H. Matsui, N. Loew et al., Air-bubble-insensitive microfluidic lactate biosensor for continuous monitoring of lactate in sweat. ACS Sens. 8(6), 2368–2374 (2023). https://doi.org/10.1021/acssensors.3c00490
J. Kim, J.R. Sempionatto, S. Imani, M.C. Hartel, A. Barfidokht et al., Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv. Sci. 5(10), 1800880 (2018). https://doi.org/10.1002/advs.201800880
M. Abu Zahed, M. Sharifuzzaman, H. Yoon, M. Asaduzzaman, D.K. Kim et al., A nanoporous carbon-MXene heterostructured nanocomposite-based epidermal patch for real-time biopotentials and sweat glucose monitoring. Adv. Funct. Mater. 32(49), 2208344 (2022). https://doi.org/10.1002/adfm.202208344
C. Xu, Y. Song, J.R. Sempionatto, S.A. Solomon, Y. Yu et al., A physicochemical-sensing electronic skin for stress response monitoring. Nat. Electron. 7(2), 168–179 (2024). https://doi.org/10.1038/s41928-023-01116-6
Z. Wang, J. Shin, J.-H. Park, H. Lee, D.-H. Kim et al., Engineering materials for electrochemical sweat sensing. Adv. Funct. Mater. 31(12), 2008130 (2021). https://doi.org/10.1002/adfm.202008130
Y. Zhao, B. Wang, H. Hojaiji, Z. Wang, S. Lin et al., A wearable freestanding electrochemical sensing system. Sci. Adv. 6(12), eaaz0007 (2020). https://doi.org/10.1126/sciadv.aaz0007
X. Huang, C. Yao, S. Huang, S. Zheng, Z. Liu et al., Technological advances of wearable device for continuous monitoring of in vivo glucose. ACS Sens. 9(3), 1065–1088 (2024). https://doi.org/10.1021/acssensors.3c01947
M. Vestergaard, K. Kerman, E. Tamiya, An overview of label-free electrochemical protein sensors. Sensors 7(12), 3442–3458 (2007). https://doi.org/10.3390/s7123442
D. Kinnamon, R. Ghanta, K.-C. Lin, S. Muthukumar, S. Prasad, Portable biosensor for monitoring Cortisol in low-volume perspired human sweat. Sci. Rep. 7(1), 13312 (2017). https://doi.org/10.1038/s41598-017-13684-7
J.S. Nah, S.C. Barman, M. Abu Zahed, M. Sharifuzzaman, H. Yoon et al., A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat Cortisol detection. Sens. Actuat. B Chem. 329, 129206 (2021). https://doi.org/10.1016/j.snb.2020.129206
S.K. Tuteja, C. Ormsby, S. Neethirajan, Noninvasive label-free detection of Cortisol and lactate using graphene embedded screen-printed electrode. Nano-Micro Lett. 10(3), 41 (2018). https://doi.org/10.1007/s40820-018-0193-5
M. Sekar, M. Pandiaraj, S. Bhansali, N. Ponpandian, C. Viswanathan, Carbon fiber based electrochemical sensor for sweat Cortisol measurement. Sci. Rep. 9(1), 403 (2019). https://doi.org/10.1038/s41598-018-37243-w
T. Laochai, J. Yukird, N. Promphet, J. Qin, O. Chailapakul et al., Non-invasive electrochemical immunosensor for sweat Cortisol based on L-cys/AuNPs/MXene modified thread electrode. Biosens. Bioelectron. 203, 114039 (2022). https://doi.org/10.1016/j.bios.2022.114039
G. Tian, Z. Zhou, M. Li, X. Li, T. Xu et al., Oriented antibody-assembled metal–organic frameworks for persistent wearable sweat Cortisol detection. Anal. Chem. 95(35), 13250–13257 (2023). https://doi.org/10.1021/acs.analchem.3c02392
S. Demuru, J. Kim, M. El Chazli, S. Bruce, M. Dupertuis et al., Antibody-coated wearable organic electrochemical transistors for Cortisol detection in human sweat. ACS Sens. 7(9), 2721–2731 (2022). https://doi.org/10.1021/acssensors.2c01250
C. Cheng, X. Li, G. Xu, Y. Lu, S.S. Low et al., Battery-free, wireless, and flexible electrochemical patch for in situ analysis of sweat Cortisol via near field communication. Biosens. Bioelectron. 172, 112782 (2021). https://doi.org/10.1016/j.bios.2020.112782
J. Aerathupalathu Janardhanan, Y.L. Chen, C.T. Liu, H.S. Tseng, P.I. Wu et al., Sensitive detection of sweat Cortisol using an organic electrochemical transistor featuring nanostructured poly(3,4-ethylenedioxythiophene) derivatives in the channel layer. Anal. Chem. 94(21), 7584–7593 (2022). https://doi.org/10.1021/acs.analchem.2c00497
H.-J. Jang, T. Lee, J. Song, L. Russell, H. Li et al., Electronic Cortisol detection using an antibody-embedded polymer coupled to a field-effect transistor. ACS Appl. Mater. Interfaces 10(19), 16233–16237 (2018). https://doi.org/10.1021/acsami.7b18855
B. Jagannath, K.-C. Lin, M. Pali, D. Sankhala, S. Muthukumar et al., A sweat-based wearable enabling technology for real-time monitoring of IL-1β and CRP as potential markers for inflammatory bowel disease. Inflamm. Bowel Dis. 26(10), 1533–1542 (2020). https://doi.org/10.1093/ibd/izaa191
K. Khachornsakkul, W. Dungchai, N. Pamme, Distance-based all-In-one immunodevice for point-of-care monitoring of cytokine interleukin-6. ACS Sens. 7(8), 2410–2419 (2022). https://doi.org/10.1021/acssensors.2c01122
R.D. Munje, S. Muthukumar, B. Jagannath, S. Prasad, A new paradigm in sweat based wearable diagnostics biosensors using Room Temperature Ionic Liquids (RTILs). Sci. Rep. 7, 1950 (2017). https://doi.org/10.1038/s41598-017-02133-0
B. Jagannath, K.-C. Lin, M. Pali, D. Sankhala, S. Muthukumar et al., Temporal profiling of cytokines in passively expressed sweat for detection of infection using wearable device. Bioeng. Transl. Med. 6(3), e10220 (2021). https://doi.org/10.1002/btm2.10220
G.S. Perera, T. Ahmed, L. Heiss, S. Walia, M. Bhaskaran et al., Rapid and selective biomarker detection with conductometric sensors. Small 17(7), 2005582 (2021). https://doi.org/10.1002/smll.202005582
T. De Meyer, S. Muyldermans, A. Depicker, Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 32(5), 263–270 (2014). https://doi.org/10.1016/j.tibtech.2014.03.001
A. Gray, A.R.M. Bradbury, A. Knappik, A. Plückthun, C.A.K. Borrebaeck et al., Animal-free alternatives and the antibody iceberg. Nat. Biotechnol. 38(11), 1234–1239 (2020). https://doi.org/10.1038/s41587-020-0687-9
J. Zheng, R. Yang, M. Shi, C. Wu, X. Fang et al., Rationally designed molecular beacons for bioanalytical and biomedical applications. Chem. Soc. Rev. 44(10), 3036–3055 (2015). https://doi.org/10.1039/C5CS00020C
C.P. Rusconi, E. Scardino, J. Layzer, G.A. Pitoc, T.L. Ortel et al., RNA aptamers as reversible antagonists of coagulation factor IXa. Nature 419(6902), 90–94 (2002). https://doi.org/10.1038/nature00963
S. Song, L. Wang, J. Li, C. Fan, J. Zhao, Aptamer-based biosensors. Trac Trends Anal. Chem. 27(2), 108–117 (2008). https://doi.org/10.1016/j.trac.2007.12.004
S. Sheibani, L. Capua, S. Kamaei, S.S.A. Akbari, J. Zhang et al., Extended gate field-effect-transistor for sensing Cortisol stress hormone. Commun. Mater. 2(1), 10 (2021). https://doi.org/10.1038/s43246-020-00114-x
M. Elbadawi, J.J. Ong, T.D. Pollard, S. Gaisford, A.W. Basit, Additive manufacturable materials for electrochemical biosensor electrodes. Adv. Funct. Mater. 31(10), 2006407 (2021). https://doi.org/10.1002/adfm.202006407
A. Ganguly, K.C. Lin, S. Muthukumar, S. Prasad, Autonomous, real-time monitoring electrochemical aptasensor for circadian tracking of Cortisol hormone in sub-microliter volumes of passively eluted human sweat. ACS Sens. 6(1), 63–72 (2021). https://doi.org/10.1021/acssensors.0c01754
M. Janghorban, I. Aradanas, K. Malaeb, H. Abuelazm, A. Nittala et al., Redox-concatenated aptamer integrated skin mimicking electrochemical patch for noninvasive detection of Cortisol. ACS Sens. 9(2), 799–809 (2024). https://doi.org/10.1021/acssensors.3c02110
N.K. Singh, S. Chung, A.-Y. Chang, J. Wang, D.A. Hall, A non-invasive wearable stress patch for real-time Cortisol monitoring using a pseudoknot-assisted aptamer. Biosens. Bioelectron. 227, 115097 (2023). https://doi.org/10.1016/j.bios.2023.115097
Z. Hao, Z. Wang, Y. Li, Y. Zhu, X. Wang et al., Measurement of cytokine biomarkers using an aptamer-based affinity graphene nanosensor on a flexible substrate toward wearable applications. Nanoscale 10(46), 21681–21688 (2018). https://doi.org/10.1039/C8NR04315A
Z. Wang, Z. Hao, X. Wang, C. Huang, Q. Lin et al., A flexible and regenerative aptameric graphene–nafion biosensor for cytokine storm biomarker monitoring in undiluted biofluids toward wearable applications. Adv. Funct. Mater. 31(4), 2005958 (2021). https://doi.org/10.1002/adfm.202005958
C. Huang, D. Li, J. Liu, S. Hou, W. Yang et al., A flexible aptameric graphene field-effect nanosensor capable of automatic liquid collection/filtering for cytokine storm biomarker monitoring in undiluted sweat. Adv. Funct. Mater. 34(9), 2309447 (2024). https://doi.org/10.1002/adfm.202309447
H. Chu, X. Hu, C.-Y. Lee, A. Zhang, Y. Ye et al., A wearable electrochemical fabric for cytokine monitoring. Biosens. Bioelectron. 232, 115301 (2023). https://doi.org/10.1016/j.bios.2023.115301
Y. Dong, T.-L. Liu, S. Chen, P. Nithianandam, K. Matar et al., A “two-part” resonance circuit based detachable sweat patch for noninvasive biochemical and biophysical sensing. Adv. Funct. Mater. 33(6), 2210136 (2023). https://doi.org/10.1002/adfm.202210136
S. Dalirirad, A.J. Steckl, Aptamer-based lateral flow assay for point of care Cortisol detection in sweat. Sens. Actuat. B Chem. 283, 79–86 (2019). https://doi.org/10.1016/j.snb.2018.11.161
L.S. Liu, F. Wang, Y. Ge, P.K. Lo, Recent developments in aptasensors for diagnostic applications. ACS Appl. Mater. Interfaces 13(8), 9329–9358 (2021). https://doi.org/10.1021/acsami.0c14788
L. Meng, A.P.F. Turner, W.C. Mak, Soft and flexible material-based affinity sensors. Biotechnol. Adv. 39, 107398 (2020). https://doi.org/10.1016/j.biotechadv.2019.05.004
S.A. Zaidi, Latest trends in molecular imprinted polymer based drug delivery systems. RSC Adv. 6(91), 88807–88819 (2016). https://doi.org/10.1039/c6ra18911c
Q. Zhang, D. Jiang, C. Xu, Y. Ge, X. Liu et al., Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat. Sens. Actuat. B Chem. 320, 128325 (2020). https://doi.org/10.1016/j.snb.2020.128325
H. Zhao, X. Zhang, Y. Qin, Y. Xia, X. Xu et al., An integrated wearable sweat sensing patch for passive continuous analysis of stress biomarkers at rest. Adv. Funct. Mater. 33(9), 2212083 (2023). https://doi.org/10.1002/adfm.202212083
A. Mani, T.S. Anirudhan, Electrochemical sensing of Cortisol by gold nanop incorporated carboxylated graphene oxide based molecularly imprinted polymer. Chem. Eng. J. 493, 152654 (2024). https://doi.org/10.1016/j.cej.2024.152654
M.-M. Chen, S.-B. Cheng, K. Ji, J. Gao, Y.-L. Liu et al., Construction of a flexible electrochemiluminescence platform for sweat detection. Chem. Sci. 10(25), 6295–6303 (2019). https://doi.org/10.1039/c9sc01937e
H. Liu, W. Qin, X. Li, L. Feng, C. Gu et al., Molecularly imprinted electrochemical sensors based on Ti3C2Tx-MXene and graphene composite modifications for ultrasensitive Cortisol detection. Anal. Chem. 95(44), 16079–16088 (2023). https://doi.org/10.1021/acs.analchem.3c01715
E. Sehit, J. Drzazgowska, D. Buchenau, C. Yesildag, M. Lensen et al., Ultrasensitive nonenzymatic electrochemical glucose sensor based on gold nanops and molecularly imprinted polymers. Biosens. Bioelectron. 165, 112432 (2020). https://doi.org/10.1016/j.bios.2020.112432
M. Singh, S. Singh, S.P. Singh, S.S. Patel, Recent advancement of carbon nanomaterials engrained molecular imprinted polymer for environmental matrix. Trends Environ. Anal. Chem. 27, e00092 (2020). https://doi.org/10.1016/j.teac.2020.e00092
L.P.C. Gomez, A. Spangenberg, X.-A. Ton, Y. Fuchs, F. Bokeloh et al., Rapid prototyping of chemical microsensors based on molecularly imprinted polymers synthesized by two-photon stereolithography. Adv. Mater. 28(28), 5931–5937 (2016). https://doi.org/10.1002/adma.201600218
X. Yang, J. Sun, F. Cui, J. Ji, L. Wang et al., An eco-friendly sensor based on CQD@MIPs for detection of N-acylated homoserine lactones and its 3D printing applications. Talanta 219, 121343 (2020). https://doi.org/10.1016/j.talanta.2020.121343
J.C. Yang, J. Lee, S.W. Hong, J. Park, Molecularly imprinted quartz crystal microbalance sensors with lithographically patterned frisbee-like pillar arrays for sensitive and selective detection of iprodione. Sens. Actuat. B Chem. 320, 128366 (2020). https://doi.org/10.1016/j.snb.2020.128366
W. Tang, L. Yin, J.R. Sempionatto, J.-M. Moon, H. Teymourian et al., Touch-based stressless Cortisol sensing. Adv. Mater. 33(18), e2008465 (2021). https://doi.org/10.1002/adma.202008465
Y. Bai, J. Fu, Z. Qin, Q. Gao, S. Li, Integrated biosensing system of electrochemistry and electrophysiology for Cortisol and skin conductance analysis on smartphone. Sens. Actuat. B Chem. 394, 134368 (2023). https://doi.org/10.1016/j.snb.2023.134368
G. Dykstra, I. Chapa, Y. Liu, Reagent-free lactate detection using Prussian blue and electropolymerized-molecularly imprinted polymers-based electrochemical biosensors. ACS Appl. Mater. Interfaces 16(49), 66921–66931 (2024). https://doi.org/10.1021/acsami.3c19448
S. Yeasmin, A. Ullah, B. Wu, X. Zhang, L.-J. Cheng, Enzyme-mimics for sensitive and selective steroid metabolite detection. ACS Appl. Mater. Interfaces (2023). https://doi.org/10.1021/acsami.2c21980
S. Yeasmin, A. Ullah, B. Wu, X. Zhang, L.-J. Cheng, Hybrid functional polymer-enabled multiplexed chemosensor patch for wearable adrenocortex stress profiling. ACS Appl. Mater. Interfaces 15(43), 50034–50046 (2023). https://doi.org/10.1021/acsami.3c11374
X. Hu, Y. Chen, X. Wang, K. Jia, H. Zhang et al., Wearable and regenerable electrochemical fabric sensing system based on molecularly imprinted polymers for real-time stress management. Adv. Funct. Mater. 34(14), 2312897 (2024). https://doi.org/10.1002/adfm.202312897
W.-T. Ting, M.-J. Wang, M.M.R. Howlader, Interleukin-6 electrochemical sensor using poly(o-phenylenediamine)-based molecularly imprinted polymer. Sens. Actuat. B Chem. 404, 135282 (2024). https://doi.org/10.1016/j.snb.2024.135282
O. Parlak, S.T. Keene, A. Marais, V.F. Curto, A. Salleo, Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive Cortisol sensing. Sci. Adv. 4(7), eaar2904 (2018). https://doi.org/10.1126/sciadv.aar2904
P. Kanokpaka, L.-Y. Chang, B.-C. Wang, T.-H. Huang, M.-J. Shih et al., Self-powered molecular imprinted polymers-based triboelectric sensor for noninvasive monitoring lactate levels in human sweat. Nano Energy 100, 107464 (2022). https://doi.org/10.1016/j.nanoen.2022.107464
S. Ramanavicius, A. Ramanavicius, Development of molecularly imprinted polymer based phase boundaries for sensors design (review). Adv. Colloid Interface Sci. 305, 102693 (2022). https://doi.org/10.1016/j.cis.2022.102693
J.J. BelBruno, Molecularly imprinted polymers. Chem. Rev. 119(1), 94–119 (2019). https://doi.org/10.1021/acs.chemrev.8b00171
K. Kaewpradub, K. Veenuttranon, H. Jantapaso, P. Mittraparp-Arthorn, I. Jeerapan, A fully-printed wearable bandage-based electrochemical sensor with pH correction for wound infection monitoring. Nano-Micro Lett. 17(1), 71 (2024). https://doi.org/10.1007/s40820