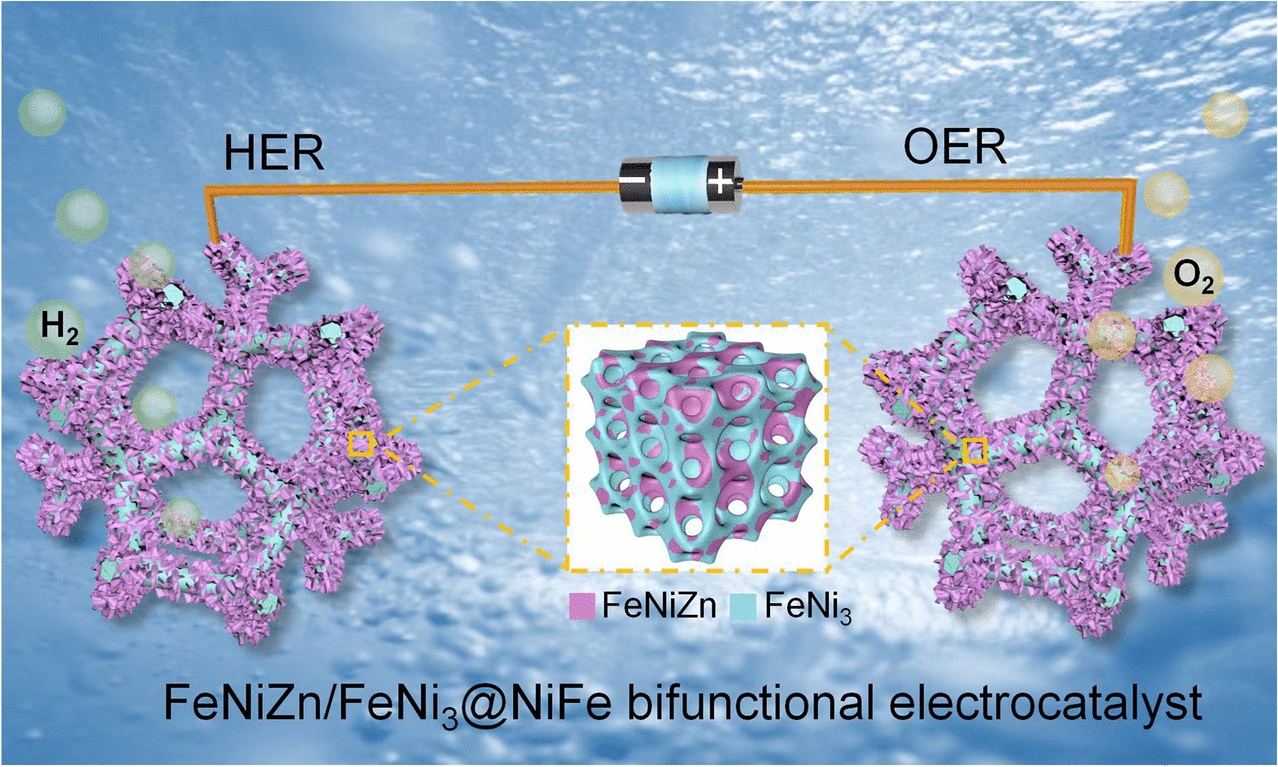
Duplex Interpenetrating-Phase FeNiZn and FeNi3 Heterostructure with Low-Gibbs Free Energy Interface Coupling for Highly Efficient Overall Water Splitting
Corresponding Author: Hong Liu
Nano-Micro Letters,
Vol. 15 (2023), Article Number: 95
Abstract
The sluggish kinetics of both hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) generate the large overpotential in water electrolysis and thus high-cost hydrogen production. Here, multidimensional nanoporous interpenetrating-phase FeNiZn alloy and FeNi3 intermetallic heterostructure is in situ constructed on NiFe foam (FeNiZn/FeNi3@NiFe) by dealloying protocol. Coupling with the eminent synergism among specific constituents and the highly efficient mass transport from integrated porous backbone, FeNiZn/FeNi3@NiFe depicts exceptional bifunctional activities for water splitting with extremely low overpotentials toward OER and HER (η1000 = 367/245 mV) as well as the robust durability during the 400 h testing in alkaline solution. The as-built water electrolyzer with FeNiZn/FeNi3@NiFe as both anode and cathode exhibits record-high performances for sustainable hydrogen output in terms of much lower cell voltage of 1.759 and 1.919 V to deliver the current density of 500 and 1000 mA cm−2 as well long working lives. Density functional theory calculations disclose that the interface interaction between FeNiZn alloy and FeNi3 intermetallic generates the modulated electron structure state and optimized intermediate chemisorption, thus diminishing the energy barriers for hydrogen production in water splitting. With the merits of fine performances, scalable fabrication, and low cost, FeNiZn/FeNi3@NiFe holds prospective application potential as the bifunctional electrocatalyst for water splitting.
Highlights:
1 Free-standing bimodal porous interpenetrating-phase FeNiZn and FeNi3 intermetallic heterostructure is in situ built on NiFe foam by dealloying way.
2 FeNiZn/FeNi3@NiFe exhibits superior electrocatalytic activities toward both oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) with very low overpotentials and prolonged catalytic durability in alkaline medium.
3 The strong synergy between FeNiZn alloy and FeNi3 intermetallic generates modulated electron structure and thus well optimizes the intermediates chemisorption toward OER and HER.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- P. Wang, Y.Z. Luo, G.X. Zhang, Z.S. Chen, H. Ranganathan et al., Interface engineering of NixSy@MnOxHy nanorods to efficiently enhance overall-water-splitting activity and stability. Nano-Micro Lett. 14, 120 (2022). https://doi.org/10.1007/s40820-022-00860-2
- R.Z. He, C.Y. Wang, L.G. Feng, Amorphous FeCoNi-S as efficient bifunctional electrocatalysts for overall water splitting reaction. Chin. Chem. Lett. 34(2), 107241 (2023). https://doi.org/10.1016/j.cclet.2022.02.046
- H.M. Sun, Z.H. Yan, F.M. Liu, W.C. Xu, F.Y. Cheng et al., Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 32(3), 1806326 (2019). https://doi.org/10.1002/adma.201806326
- J.L. Zhu, J.M. Qian, X.B. Peng, B.R. Xia, D.Q. Gao, Etching-induced surface reconstruction of NiMoO4 for oxygen evolution reaction. Nano-Micro Lett. 15, 30 (2023). https://doi.org/10.1007/s40820-022-01011-3
- C. Walter, P.W. Menezes, M. Driess, Perspective on intermetallics towards efficient electrocatalytic water-splitting. Chem. Sci. 12, 8603–8631 (2021). https://doi.org/10.1039/D1SC01901E
- H.Y. Kim, S.H. Joo, Recent advances in nanostructured intermetallic electrocatalysts for renewable energy conversion reactions. J. Mater. Chem. A 8, 8195–8217 (2020). https://doi.org/10.1039/d0ta01809k
- J.R. Li, S.H. Sun, Intermetallic nanops: synthetic control and their enhanced electrocatalysis. Acc. Chem. Res. 52(7), 2015–2025 (2019). https://doi.org/10.1021/acs.accounts.9b00172
- A. Delvaux, Q. Van Overmeere, R. Poulain, J. Proost, Enhanced oxygen evolution during water electrolysis at de-alloyed nickel thin film electrodes. J. Electrochem. Soc. 164, F1196–F1203 (2017). https://doi.org/10.1149/2.1451712jes
- Y. Jin, X. Yue, C. Shu, S.L. Huang, P.K. Shen, Three-dimensional porous MoNi4 networks constructed by nanosheets as bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 5, 2508–2513 (2017). https://doi.org/10.1039/C6TA10802D
- C.A. Ding, J.W. Zhu, X.Q. Mu, R.L. Cheng, W.Q. Li et al., Nitrogen-doped carbon coupled FeNi3 intermetallic compound as advanced bifunctional electrocatalyst for OER, ORR and Zn-air batteries. Appl. Catal. B Environ. 268, 118729 (2020). https://doi.org/10.1016/j.apcatb.2020.118729
- K. Chen, S. Kim, R. Rajendiran, K. Prabakar, G.Z. Li et al., Enhancing ORR/OER active sites through lattice distortion of Fe-enriched FeNi3 intermetallic nanops doped N-doped carbon for high-performance rechargeable Zn-air battery. J. Colloid Interface Sci. 582, 977–990 (2021). https://doi.org/10.1016/j.jcis.2020.08.101
- Y. Zhu, Y. Chen, Y. Zhong, Y.B. Chen, N.N. Ma et al., A surface-modified antiperovskite as an electrocatalyst for water oxidation. Nat. Commun. 9, 2326 (2018). https://doi.org/10.1038/s41467-018-04682-y
- L. Rößner, H. Schwarz, I. Veremchuk, R. Zerdoumi, T. Seyller et al., Challenging the durability of intermetallic Mo-Ni compounds in the hydrogen evolution reaction. ACS Appl. Mater. Interfaces 13(20), 23616–23626 (2021). https://doi.org/10.1021/acsami.1c02169
- J. Zhang, T. Wang, P. Liu, Z.Q. Liao, S.H. Liu et al., Efficient hydrogen production on MoNi4 electrocatalysts with fast water dissociation kinetics. Nat. Commun. 8, 15437 (2017). https://doi.org/10.1038/ncomms15437
- N.V. Krstajic, B.N. Grgur, M. Zdujic, M.V. Vojnović, M.M. Jakšićc, Kinetic properties of the Ti-Ni intermetallic phases and alloys for hydrogen evolution. J. Alloys Compd. 257(1–2), 245–252 (1997). https://doi.org/10.1016/S0925-8388(97)00018-2
- N.V. Krstajic, B.N. Grgur, N.S. Mladenovic, M.V. Vojnović, M.M. Jaks̆ić, The determination of kinetics parameters of the hydrogen evolution on Ti-Ni alloys by ac impedance. Electrochim. Acta 42(2), 323–330 (1997). https://doi.org/10.1016/S0360-3199(99)00075-0
- Z. Zheng, N. Li, C.Q. Wang, D.Y. Li, F.Y. Meng et al., Effects of CeO2 on the microstructure and hydrogen evolution property of Ni-Zn coatings. J. Power Sources 222, 88–91 (2013). https://doi.org/10.1016/j.jpowsour.2012.08.077
- Q.X. Zhou, Q. Hao, Y.X. Li, J.H. Yu, C.X. Xu et al., Free-standing trimodal porous NiZn intermetallic and Ni heterojunction as highly efficient hydrogen evolution electrocatalyst in the alkaline electrolyte. Nano Energy 89, 106402 (2021). https://doi.org/10.1016/j.nanoen.2021.106402
- Z.J. Li, H. Jang, D.N. Qin, X.L. Jiang, X.Q. Ji et al., Alloy-strain-output induced lattice dislocation in Ni3FeN/Ni3Fe ultrathin nanosheets for highly efficient overall water splitting. J. Mater. Chem. A 9, 4036–4043 (2021). https://doi.org/10.1039/D0TA11618A
- S.Q. Liang, M.Z. Jing, T.J. Thomas, J. Liu, H.C. Guo et al., FeNi3-FeNi3N- a high-performance catalyst for overall water splitting. Sustain. Energy Fuels 4, 6245–6250 (2020). https://doi.org/10.1039/D0SE01491E
- H.M. Sun, C.Y. Tian, G.L. Fan, J.N. Qi, Z.T. Liu et al., Boosting activity on Co4N porous nanosheet by coupling CeO2 for efficient electrochemical overall water splitting at high current densities. Adv. Funct. Mater. 30(32), 1910596 (2020). https://doi.org/10.1002/adfm.201910596
- Z.Z. Yin, R.Z. He, Y.C. Zhang, L.G. Feng, X. Wu et al., Electrochemical deposited amorphous FeNi hydroxide electrode for oxygen evolution reaction. J. Energy Chem. 69, 585–592 (2022). https://doi.org/10.1016/j.jechem.2022.01.020
- J.H. Lin, Y.T. Yan, C. Li, X.Q. Si, H.H. Wang et al., Bifunctional electrocatalysts based on Mo-doped NiCoP nanosheet arrays for overall water splitting. Nano-Micro Lett. 11, 55 (2019). https://doi.org/10.1007/s40820-019-0289-6
- A. Qayum, X. Peng, J.F. Yuan, Y.D. Qu, J.H. Zhou et al., Highly durable and efficient Ni-FeOx/FeNi3 electrocatalysts synthesized by a facile in situ combustion-based method for overall water splitting with large current densities. ACS Appl. Mater. Interfaces 14(24), 27842–27853 (2022). https://doi.org/10.1021/acsami.2c04562
- H. Zhang, G.F. Qian, T.Q. Yu, J.L. Chen, L. Luo et al., Interface engineering of Ni3Fe and FeV2O4 coupling with carbon coated mesoporous nanosheets for boosting overall water splitting at 1500 mA cm−2. ACS Sustain. Chem. Eng. 9(24), 8249–8256 (2021). https://doi.org/10.1021/acssuschemeng.1c02293
- W.M. Wang, G.F. Qian, Q.L. Xu, H. Zhang, F. Shen et al., Industrially promising IrNi-FeNi3 hybrid nanosheets for overall water splitting catalysis at large current density. Appl. Catal. B Environ. 286, 119881 (2021). https://doi.org/10.1016/j.apcatb.2021.119881
- W. Luc, F. Jiao, Synthesis of nanoporous metals, oxides, carbides, and sulfides: beyond nanocasting. Acc. Chem. Res. 49(7), 1351–1358 (2016). https://doi.org/10.1021/acs.accounts.6b00109
- J.W. Li, W.M. Xu, J.X. Luo, D. Zhou, D.W. Zhang et al., Synthesis of 3D hexagram-like cobalt-manganese sulfides nanosheets grown on nickel foam: a bifunctional electrocatalyst for overall water splitting. Nano-Micro Lett. 10, 6 (2018). https://doi.org/10.1007/s40820-017-0160-6
- W. Du, Y.M. Shi, W. Zhou, Y.F. Yu, B. Zhang, Unveiling the in situ dissolution and polymerization of Mo in Ni4Mo alloy for promoting the hydrogen evolution reaction. Angew. Chem. Int. Ed. 60(13), 7051–7055 (2021). https://doi.org/10.1002/anie.202015723
- Y.H. Wu, Y. Zhang, Y.X. Wang, Z. He, Z.J. Gu et al., Potentiostatic electrodeposited of Ni-Fe-Sn on Ni foam served as an excellent electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 46, 26930–26939 (2021). https://doi.org/10.1016/j.ijhydene.2021.05.189
- X.X. Zhang, J. Wang, J.Y. Wang, J.Z. Wang, C.W. Wang et al., Freestanding surface disordered NiCu solid solution as ultrastable high current density hydrogen evolution reaction electrode. J. Phys. Chem. Lett. 12(45), 11135–11142 (2021). https://doi.org/10.1021/acs.jpclett.1c03041
- G. Kresse, J. Furthmüller, Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996). https://doi.org/10.1016/0927-0256(96)00008-0
- J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). https://doi.org/10.1103/PhysRevLett.77.3865
- P.E. Blöchl, Projected augmented-wave method. Phys. Rev. B Condens. Matter 50, 17953–17979 (1994). https://doi.org/10.1103/physrevb.50.17953
- G. Kresse, D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999). https://doi.org/10.1103/PhysRevB.59.1758
- C. Tsai, K. Chan, J.K. Nørskov, F. Abild-Pedersen, Rational design of MoS2 catalysts: tuning the structure and activity via transition metal doping. Catal. Sci. Technol. 5, 246–253 (2015). https://doi.org/10.1103/PhysRevB.59.1758
- W.H. Dai, L. Li, Y.M. Li, Z. Chen, F. Liu et al., A novel Ni-S-Mn electrode with hierarchical morphology fabricated by gradient electrodeposition for hydrogen evolution reaction. Appl. Surf. Sci. 514, 145944 (2020). https://doi.org/10.1016/j.apsusc.2020.145944
- J.Q. Lian, Y.H. Wu, J.J. Sun, High current density electrodeposition of NiFe/nickel foam as a bifunctional electrocatalyst for overall water splitting in alkaline electrolyte. J. Mater. Sci. 55, 15140–15151 (2020). https://doi.org/10.1007/s10853-020-05080-w
- Y.Y. Wu, R. Su, Y. Li, Z.H. Wang, Z. Lü et al., Redox sculptured dual-scale porous nickel-iron foams for efficient water oxidation. Electrochim. Acta 309, 415–423 (2019). https://doi.org/10.1016/j.electacta.2019.04.065
- Y.J. Zhou, Y.H. Lia, L.X. Zhang, L.L. Zhang, L. Li et al., Fe-leaching induced surface reconstruction of Ni-Fe alloy on N-doped carbon to boost oxygen evolution reaction. Chem. Eng. J. 394, 124977 (2020). https://doi.org/10.1016/j.cej.2020.124977
- H. Zhang, B.J. Xi, Y. Gu, W.H. Chen, S.L. Xiong, Interface engineering and heterometal doping Mo-NiS/Ni(OH)2 for overall water splitting. Nano Res. 14, 3466–3473 (2021). https://doi.org/10.1007/s12274-021-3557-y
- J. Zhang, T. Wang, D. Pohl, B. Rellinghaus, R. Dong et al., Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overall-water-splitting activity. Angew. Chem. Int. Ed. 55(23), 6702–6707 (2016). https://doi.org/10.1002/anie.201602237
- J. Huang, P.L. Wang, P. Li, H.Y. Yin, D.H. Wang, Regulating electrolytic Fe0.5CoNiCuZnx high entropy alloy electrodes for oxygen evolution reactions in alkaline solution. J. Mater. Sci. Technol. 93, 110–118 (2021). https://doi.org/10.1016/j.jmst.2021.03.046
- H. Liu, C. Xi, J.D. Xin, G.L. Zhang, S.F. Zhang et al., Free-standing nanoporous NiMnFeMo alloy: an efficient non-precious metal electrocatalyst for water splitting. Chem. Eng. J. 404, 126530 (2021). https://doi.org/10.1016/j.cej.2020.126530
- P. Zhang, L. Li, D. Nordlund, H. Chen, L.Z. Fan et al., Dendritic core-shell nickel-iron-copper metal/metal oxide electrode for efficient electrocatalytic water oxidation. Nat. Commun. 9, 381 (2018). https://doi.org/10.1038/s41467-017-02429-9
- B.S. Yeo, A.T. Bell, In situ Raman study of nickel oxide and gold-supported nickel oxide catalysts for the electrochemical evolution of oxygen. J. Phys. Chem. C 116(15), 8394–8400 (2012). https://doi.org/10.1021/jp3007415
- Z. Li, X.L. Xu, X.H. Lu, C.C. He, J.J. Huang et al., Synergistic coupling of FeNi3 alloy with graphene carbon dots for advanced oxygen evolution reaction electrocatalysis. J. Colloid Interface Sci. 615, 273–281 (2022). https://doi.org/10.1016/j.jcis.2022.01.088
- X. Teng, J.Y. Wang, L.L. Ji, Y.Y. Liu, C. Zhang et al., Fabrication of three-dimensional multiscale porous alloy foams at a planar substrate for efficient water splitting. ACS Sustain. Chem. Eng. 7(5), 5412–5419 (2019). https://doi.org/10.1021/acssuschemeng.8b06452
- Q. Li, W.X. Zhang, J. Shen, X.Y. Zhang, Z. Liu et al., Trimetallic nanoplate arrays of Ni-Fe-Mo sulfide on FeNi3 foam: a highly efficient and bifunctional electrocatalyst for overall water splitting. J. Alloys Compd. 902, 163670 (2022). https://doi.org/10.1016/j.jallcom.2022.163670
- C.L. Huang, X.F. Chuah, C.T. Hsieh, S.Y. Lu, NiFe alloy nanotube arrays as highly efficient bifunctional electrocatalysts for overall water splitting at high current densities. ACS Appl. Mater. Interfaces 11(27), 24096–24106 (2019). https://doi.org/10.1021/acsami.9b05919
- Z.H. Liu, S.F. Li, F.F. Wang, M.X. Li, Y.H. Ni, Hierarchically porous FeNi3@FeNi layered double hydroxide nanostructures: one-step fast electrodeposition and highly efficient electrocatalytic performances for overall water splitting. Dalton Trans. 50, 6306–6314 (2021). https://doi.org/10.1039/D0DT04366D
- P. Rasiyah, A.C.C. Tseung, The role of the lower metal oxide/higher metal oxide couple in oxygen evolution reactions. J. Electrochem. Soc. 131(4), 803 (1984). https://doi.org/10.1149/1.2115703
- L. Xu, F.T. Zhang, J.G. Chen, X.Z. Fu, R. Sun et al., Amorphous NiFe nanotube arrays bifunctional electrocatalysts for efficient electrochemical overall water splitting. ACS Appl. Energy Mater. 1(3), 1210–1217 (2018). https://doi.org/10.1021/acsaem.7b00313
- Y.J. Li, Z.F. Mao, Q. Wang, D.B. Li, R. Wang et al., Hollow nanosheet array of phosphorus-anion-decorated cobalt disulfide as an efficient electrocatalyst for overall water splitting. Chem. Eng. J. 390, 124556 (2020). https://doi.org/10.1016/j.cej.2020.124556
- J. Tian, Q. Liu, A.M. Asiri, X.P. Sun, Self-supported nanoporous cobalt phosphide nanowire arrays: an efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 136(21), 7587–7590 (2014). https://doi.org/10.1021/ja503372r
- J. Shen, Q. Li, W.X. Zhang, Z.Y. Cai, L. Cui et al., Spherical Co3S4 grown directly on Ni-Fe sulfides as a porous nanoplate array on FeNi3 foam: a highly efficient and durable bifunctional catalyst for overall water splitting. J. Mater. Chem. A 10, 5442 (2020). https://doi.org/10.1039/d1ta07504g
- W.X. Zhang, Q. Jia, H. Liang, L. Cui, D. Wei et al., Iron doped Ni3S2 nanorods directly grown on FeNi3 foam as an efficient bifunctional catalyst for overall water splitting. Chem. Eng. J. 396, 125315 (2020). https://doi.org/10.1016/j.cej.2020.125315
- X.D. Yan, L.H. Tian, K.X. Li, S. Atkins, H.F. Zhao et al., FeNi3/NiFeOx nanohybrids as highly efficient bifunctional electrocatalysts for overall water splitting. Adv. Mater. Interfaces 3(22), 1600368 (2016). https://doi.org/10.1002/admi.201600368
- S.F. Sun, X. Zhou, B.W. Cong, W. Hong, G. Chen, Tailoring the d-band centers endows (NixFe1–x)2P nanosheets with efficient oxygen evolution catalysis. ACS Catal. 10(16), 9086–9097 (2020). https://doi.org/10.1021/acscatal.0c01273
- V.R. Stamenkovic, B.S. Mun, M. Arenz, K.J.J. Mayrhofer, C.A. Lucas et al., Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 6, 241–247 (2007). https://doi.org/10.1038/nmat1840
- J.Q. Yan, L.Q. Kong, Y.J. Ji, J. White, Y.Y. Li et al., Single atom tungsten doped ultrathin α-Ni(OH)2 for enhanced electrocatalytic water oxidation. Nat. Commun. 10, 2149 (2019). https://doi.org/10.1038/s41467-019-09845-z
- Q.M. Chen, N. Gong, T.R. Zhu, C.Y. Yang, W.C. Peng et al., Surface phase engineering modulated iron-nickel nitrides/alloy nanospheres with tailored d-band center for efficient oxygen evolution reaction. Small 18(4), 2105696 (2022). https://doi.org/10.1002/smll.202105696
- Z.Y. Chen, Y. Song, J.Y. Cai, X.S. Zheng, D.D. Han et al., Tailoring the d-band centers enables Co4N nanosheets to be highly active for hydrogen evolution catalysis. Angew. Chem. Int. Ed. 57(18), 5076–5080 (2018). https://doi.org/10.1002/anie.201801834
References
P. Wang, Y.Z. Luo, G.X. Zhang, Z.S. Chen, H. Ranganathan et al., Interface engineering of NixSy@MnOxHy nanorods to efficiently enhance overall-water-splitting activity and stability. Nano-Micro Lett. 14, 120 (2022). https://doi.org/10.1007/s40820-022-00860-2
R.Z. He, C.Y. Wang, L.G. Feng, Amorphous FeCoNi-S as efficient bifunctional electrocatalysts for overall water splitting reaction. Chin. Chem. Lett. 34(2), 107241 (2023). https://doi.org/10.1016/j.cclet.2022.02.046
H.M. Sun, Z.H. Yan, F.M. Liu, W.C. Xu, F.Y. Cheng et al., Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 32(3), 1806326 (2019). https://doi.org/10.1002/adma.201806326
J.L. Zhu, J.M. Qian, X.B. Peng, B.R. Xia, D.Q. Gao, Etching-induced surface reconstruction of NiMoO4 for oxygen evolution reaction. Nano-Micro Lett. 15, 30 (2023). https://doi.org/10.1007/s40820-022-01011-3
C. Walter, P.W. Menezes, M. Driess, Perspective on intermetallics towards efficient electrocatalytic water-splitting. Chem. Sci. 12, 8603–8631 (2021). https://doi.org/10.1039/D1SC01901E
H.Y. Kim, S.H. Joo, Recent advances in nanostructured intermetallic electrocatalysts for renewable energy conversion reactions. J. Mater. Chem. A 8, 8195–8217 (2020). https://doi.org/10.1039/d0ta01809k
J.R. Li, S.H. Sun, Intermetallic nanops: synthetic control and their enhanced electrocatalysis. Acc. Chem. Res. 52(7), 2015–2025 (2019). https://doi.org/10.1021/acs.accounts.9b00172
A. Delvaux, Q. Van Overmeere, R. Poulain, J. Proost, Enhanced oxygen evolution during water electrolysis at de-alloyed nickel thin film electrodes. J. Electrochem. Soc. 164, F1196–F1203 (2017). https://doi.org/10.1149/2.1451712jes
Y. Jin, X. Yue, C. Shu, S.L. Huang, P.K. Shen, Three-dimensional porous MoNi4 networks constructed by nanosheets as bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 5, 2508–2513 (2017). https://doi.org/10.1039/C6TA10802D
C.A. Ding, J.W. Zhu, X.Q. Mu, R.L. Cheng, W.Q. Li et al., Nitrogen-doped carbon coupled FeNi3 intermetallic compound as advanced bifunctional electrocatalyst for OER, ORR and Zn-air batteries. Appl. Catal. B Environ. 268, 118729 (2020). https://doi.org/10.1016/j.apcatb.2020.118729
K. Chen, S. Kim, R. Rajendiran, K. Prabakar, G.Z. Li et al., Enhancing ORR/OER active sites through lattice distortion of Fe-enriched FeNi3 intermetallic nanops doped N-doped carbon for high-performance rechargeable Zn-air battery. J. Colloid Interface Sci. 582, 977–990 (2021). https://doi.org/10.1016/j.jcis.2020.08.101
Y. Zhu, Y. Chen, Y. Zhong, Y.B. Chen, N.N. Ma et al., A surface-modified antiperovskite as an electrocatalyst for water oxidation. Nat. Commun. 9, 2326 (2018). https://doi.org/10.1038/s41467-018-04682-y
L. Rößner, H. Schwarz, I. Veremchuk, R. Zerdoumi, T. Seyller et al., Challenging the durability of intermetallic Mo-Ni compounds in the hydrogen evolution reaction. ACS Appl. Mater. Interfaces 13(20), 23616–23626 (2021). https://doi.org/10.1021/acsami.1c02169
J. Zhang, T. Wang, P. Liu, Z.Q. Liao, S.H. Liu et al., Efficient hydrogen production on MoNi4 electrocatalysts with fast water dissociation kinetics. Nat. Commun. 8, 15437 (2017). https://doi.org/10.1038/ncomms15437
N.V. Krstajic, B.N. Grgur, M. Zdujic, M.V. Vojnović, M.M. Jakšićc, Kinetic properties of the Ti-Ni intermetallic phases and alloys for hydrogen evolution. J. Alloys Compd. 257(1–2), 245–252 (1997). https://doi.org/10.1016/S0925-8388(97)00018-2
N.V. Krstajic, B.N. Grgur, N.S. Mladenovic, M.V. Vojnović, M.M. Jaks̆ić, The determination of kinetics parameters of the hydrogen evolution on Ti-Ni alloys by ac impedance. Electrochim. Acta 42(2), 323–330 (1997). https://doi.org/10.1016/S0360-3199(99)00075-0
Z. Zheng, N. Li, C.Q. Wang, D.Y. Li, F.Y. Meng et al., Effects of CeO2 on the microstructure and hydrogen evolution property of Ni-Zn coatings. J. Power Sources 222, 88–91 (2013). https://doi.org/10.1016/j.jpowsour.2012.08.077
Q.X. Zhou, Q. Hao, Y.X. Li, J.H. Yu, C.X. Xu et al., Free-standing trimodal porous NiZn intermetallic and Ni heterojunction as highly efficient hydrogen evolution electrocatalyst in the alkaline electrolyte. Nano Energy 89, 106402 (2021). https://doi.org/10.1016/j.nanoen.2021.106402
Z.J. Li, H. Jang, D.N. Qin, X.L. Jiang, X.Q. Ji et al., Alloy-strain-output induced lattice dislocation in Ni3FeN/Ni3Fe ultrathin nanosheets for highly efficient overall water splitting. J. Mater. Chem. A 9, 4036–4043 (2021). https://doi.org/10.1039/D0TA11618A
S.Q. Liang, M.Z. Jing, T.J. Thomas, J. Liu, H.C. Guo et al., FeNi3-FeNi3N- a high-performance catalyst for overall water splitting. Sustain. Energy Fuels 4, 6245–6250 (2020). https://doi.org/10.1039/D0SE01491E
H.M. Sun, C.Y. Tian, G.L. Fan, J.N. Qi, Z.T. Liu et al., Boosting activity on Co4N porous nanosheet by coupling CeO2 for efficient electrochemical overall water splitting at high current densities. Adv. Funct. Mater. 30(32), 1910596 (2020). https://doi.org/10.1002/adfm.201910596
Z.Z. Yin, R.Z. He, Y.C. Zhang, L.G. Feng, X. Wu et al., Electrochemical deposited amorphous FeNi hydroxide electrode for oxygen evolution reaction. J. Energy Chem. 69, 585–592 (2022). https://doi.org/10.1016/j.jechem.2022.01.020
J.H. Lin, Y.T. Yan, C. Li, X.Q. Si, H.H. Wang et al., Bifunctional electrocatalysts based on Mo-doped NiCoP nanosheet arrays for overall water splitting. Nano-Micro Lett. 11, 55 (2019). https://doi.org/10.1007/s40820-019-0289-6
A. Qayum, X. Peng, J.F. Yuan, Y.D. Qu, J.H. Zhou et al., Highly durable and efficient Ni-FeOx/FeNi3 electrocatalysts synthesized by a facile in situ combustion-based method for overall water splitting with large current densities. ACS Appl. Mater. Interfaces 14(24), 27842–27853 (2022). https://doi.org/10.1021/acsami.2c04562
H. Zhang, G.F. Qian, T.Q. Yu, J.L. Chen, L. Luo et al., Interface engineering of Ni3Fe and FeV2O4 coupling with carbon coated mesoporous nanosheets for boosting overall water splitting at 1500 mA cm−2. ACS Sustain. Chem. Eng. 9(24), 8249–8256 (2021). https://doi.org/10.1021/acssuschemeng.1c02293
W.M. Wang, G.F. Qian, Q.L. Xu, H. Zhang, F. Shen et al., Industrially promising IrNi-FeNi3 hybrid nanosheets for overall water splitting catalysis at large current density. Appl. Catal. B Environ. 286, 119881 (2021). https://doi.org/10.1016/j.apcatb.2021.119881
W. Luc, F. Jiao, Synthesis of nanoporous metals, oxides, carbides, and sulfides: beyond nanocasting. Acc. Chem. Res. 49(7), 1351–1358 (2016). https://doi.org/10.1021/acs.accounts.6b00109
J.W. Li, W.M. Xu, J.X. Luo, D. Zhou, D.W. Zhang et al., Synthesis of 3D hexagram-like cobalt-manganese sulfides nanosheets grown on nickel foam: a bifunctional electrocatalyst for overall water splitting. Nano-Micro Lett. 10, 6 (2018). https://doi.org/10.1007/s40820-017-0160-6
W. Du, Y.M. Shi, W. Zhou, Y.F. Yu, B. Zhang, Unveiling the in situ dissolution and polymerization of Mo in Ni4Mo alloy for promoting the hydrogen evolution reaction. Angew. Chem. Int. Ed. 60(13), 7051–7055 (2021). https://doi.org/10.1002/anie.202015723
Y.H. Wu, Y. Zhang, Y.X. Wang, Z. He, Z.J. Gu et al., Potentiostatic electrodeposited of Ni-Fe-Sn on Ni foam served as an excellent electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 46, 26930–26939 (2021). https://doi.org/10.1016/j.ijhydene.2021.05.189
X.X. Zhang, J. Wang, J.Y. Wang, J.Z. Wang, C.W. Wang et al., Freestanding surface disordered NiCu solid solution as ultrastable high current density hydrogen evolution reaction electrode. J. Phys. Chem. Lett. 12(45), 11135–11142 (2021). https://doi.org/10.1021/acs.jpclett.1c03041
G. Kresse, J. Furthmüller, Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996). https://doi.org/10.1016/0927-0256(96)00008-0
J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). https://doi.org/10.1103/PhysRevLett.77.3865
P.E. Blöchl, Projected augmented-wave method. Phys. Rev. B Condens. Matter 50, 17953–17979 (1994). https://doi.org/10.1103/physrevb.50.17953
G. Kresse, D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999). https://doi.org/10.1103/PhysRevB.59.1758
C. Tsai, K. Chan, J.K. Nørskov, F. Abild-Pedersen, Rational design of MoS2 catalysts: tuning the structure and activity via transition metal doping. Catal. Sci. Technol. 5, 246–253 (2015). https://doi.org/10.1103/PhysRevB.59.1758
W.H. Dai, L. Li, Y.M. Li, Z. Chen, F. Liu et al., A novel Ni-S-Mn electrode with hierarchical morphology fabricated by gradient electrodeposition for hydrogen evolution reaction. Appl. Surf. Sci. 514, 145944 (2020). https://doi.org/10.1016/j.apsusc.2020.145944
J.Q. Lian, Y.H. Wu, J.J. Sun, High current density electrodeposition of NiFe/nickel foam as a bifunctional electrocatalyst for overall water splitting in alkaline electrolyte. J. Mater. Sci. 55, 15140–15151 (2020). https://doi.org/10.1007/s10853-020-05080-w
Y.Y. Wu, R. Su, Y. Li, Z.H. Wang, Z. Lü et al., Redox sculptured dual-scale porous nickel-iron foams for efficient water oxidation. Electrochim. Acta 309, 415–423 (2019). https://doi.org/10.1016/j.electacta.2019.04.065
Y.J. Zhou, Y.H. Lia, L.X. Zhang, L.L. Zhang, L. Li et al., Fe-leaching induced surface reconstruction of Ni-Fe alloy on N-doped carbon to boost oxygen evolution reaction. Chem. Eng. J. 394, 124977 (2020). https://doi.org/10.1016/j.cej.2020.124977
H. Zhang, B.J. Xi, Y. Gu, W.H. Chen, S.L. Xiong, Interface engineering and heterometal doping Mo-NiS/Ni(OH)2 for overall water splitting. Nano Res. 14, 3466–3473 (2021). https://doi.org/10.1007/s12274-021-3557-y
J. Zhang, T. Wang, D. Pohl, B. Rellinghaus, R. Dong et al., Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overall-water-splitting activity. Angew. Chem. Int. Ed. 55(23), 6702–6707 (2016). https://doi.org/10.1002/anie.201602237
J. Huang, P.L. Wang, P. Li, H.Y. Yin, D.H. Wang, Regulating electrolytic Fe0.5CoNiCuZnx high entropy alloy electrodes for oxygen evolution reactions in alkaline solution. J. Mater. Sci. Technol. 93, 110–118 (2021). https://doi.org/10.1016/j.jmst.2021.03.046
H. Liu, C. Xi, J.D. Xin, G.L. Zhang, S.F. Zhang et al., Free-standing nanoporous NiMnFeMo alloy: an efficient non-precious metal electrocatalyst for water splitting. Chem. Eng. J. 404, 126530 (2021). https://doi.org/10.1016/j.cej.2020.126530
P. Zhang, L. Li, D. Nordlund, H. Chen, L.Z. Fan et al., Dendritic core-shell nickel-iron-copper metal/metal oxide electrode for efficient electrocatalytic water oxidation. Nat. Commun. 9, 381 (2018). https://doi.org/10.1038/s41467-017-02429-9
B.S. Yeo, A.T. Bell, In situ Raman study of nickel oxide and gold-supported nickel oxide catalysts for the electrochemical evolution of oxygen. J. Phys. Chem. C 116(15), 8394–8400 (2012). https://doi.org/10.1021/jp3007415
Z. Li, X.L. Xu, X.H. Lu, C.C. He, J.J. Huang et al., Synergistic coupling of FeNi3 alloy with graphene carbon dots for advanced oxygen evolution reaction electrocatalysis. J. Colloid Interface Sci. 615, 273–281 (2022). https://doi.org/10.1016/j.jcis.2022.01.088
X. Teng, J.Y. Wang, L.L. Ji, Y.Y. Liu, C. Zhang et al., Fabrication of three-dimensional multiscale porous alloy foams at a planar substrate for efficient water splitting. ACS Sustain. Chem. Eng. 7(5), 5412–5419 (2019). https://doi.org/10.1021/acssuschemeng.8b06452
Q. Li, W.X. Zhang, J. Shen, X.Y. Zhang, Z. Liu et al., Trimetallic nanoplate arrays of Ni-Fe-Mo sulfide on FeNi3 foam: a highly efficient and bifunctional electrocatalyst for overall water splitting. J. Alloys Compd. 902, 163670 (2022). https://doi.org/10.1016/j.jallcom.2022.163670
C.L. Huang, X.F. Chuah, C.T. Hsieh, S.Y. Lu, NiFe alloy nanotube arrays as highly efficient bifunctional electrocatalysts for overall water splitting at high current densities. ACS Appl. Mater. Interfaces 11(27), 24096–24106 (2019). https://doi.org/10.1021/acsami.9b05919
Z.H. Liu, S.F. Li, F.F. Wang, M.X. Li, Y.H. Ni, Hierarchically porous FeNi3@FeNi layered double hydroxide nanostructures: one-step fast electrodeposition and highly efficient electrocatalytic performances for overall water splitting. Dalton Trans. 50, 6306–6314 (2021). https://doi.org/10.1039/D0DT04366D
P. Rasiyah, A.C.C. Tseung, The role of the lower metal oxide/higher metal oxide couple in oxygen evolution reactions. J. Electrochem. Soc. 131(4), 803 (1984). https://doi.org/10.1149/1.2115703
L. Xu, F.T. Zhang, J.G. Chen, X.Z. Fu, R. Sun et al., Amorphous NiFe nanotube arrays bifunctional electrocatalysts for efficient electrochemical overall water splitting. ACS Appl. Energy Mater. 1(3), 1210–1217 (2018). https://doi.org/10.1021/acsaem.7b00313
Y.J. Li, Z.F. Mao, Q. Wang, D.B. Li, R. Wang et al., Hollow nanosheet array of phosphorus-anion-decorated cobalt disulfide as an efficient electrocatalyst for overall water splitting. Chem. Eng. J. 390, 124556 (2020). https://doi.org/10.1016/j.cej.2020.124556
J. Tian, Q. Liu, A.M. Asiri, X.P. Sun, Self-supported nanoporous cobalt phosphide nanowire arrays: an efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 136(21), 7587–7590 (2014). https://doi.org/10.1021/ja503372r
J. Shen, Q. Li, W.X. Zhang, Z.Y. Cai, L. Cui et al., Spherical Co3S4 grown directly on Ni-Fe sulfides as a porous nanoplate array on FeNi3 foam: a highly efficient and durable bifunctional catalyst for overall water splitting. J. Mater. Chem. A 10, 5442 (2020). https://doi.org/10.1039/d1ta07504g
W.X. Zhang, Q. Jia, H. Liang, L. Cui, D. Wei et al., Iron doped Ni3S2 nanorods directly grown on FeNi3 foam as an efficient bifunctional catalyst for overall water splitting. Chem. Eng. J. 396, 125315 (2020). https://doi.org/10.1016/j.cej.2020.125315
X.D. Yan, L.H. Tian, K.X. Li, S. Atkins, H.F. Zhao et al., FeNi3/NiFeOx nanohybrids as highly efficient bifunctional electrocatalysts for overall water splitting. Adv. Mater. Interfaces 3(22), 1600368 (2016). https://doi.org/10.1002/admi.201600368
S.F. Sun, X. Zhou, B.W. Cong, W. Hong, G. Chen, Tailoring the d-band centers endows (NixFe1–x)2P nanosheets with efficient oxygen evolution catalysis. ACS Catal. 10(16), 9086–9097 (2020). https://doi.org/10.1021/acscatal.0c01273
V.R. Stamenkovic, B.S. Mun, M. Arenz, K.J.J. Mayrhofer, C.A. Lucas et al., Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 6, 241–247 (2007). https://doi.org/10.1038/nmat1840
J.Q. Yan, L.Q. Kong, Y.J. Ji, J. White, Y.Y. Li et al., Single atom tungsten doped ultrathin α-Ni(OH)2 for enhanced electrocatalytic water oxidation. Nat. Commun. 10, 2149 (2019). https://doi.org/10.1038/s41467-019-09845-z
Q.M. Chen, N. Gong, T.R. Zhu, C.Y. Yang, W.C. Peng et al., Surface phase engineering modulated iron-nickel nitrides/alloy nanospheres with tailored d-band center for efficient oxygen evolution reaction. Small 18(4), 2105696 (2022). https://doi.org/10.1002/smll.202105696
Z.Y. Chen, Y. Song, J.Y. Cai, X.S. Zheng, D.D. Han et al., Tailoring the d-band centers enables Co4N nanosheets to be highly active for hydrogen evolution catalysis. Angew. Chem. Int. Ed. 57(18), 5076–5080 (2018). https://doi.org/10.1002/anie.201801834