Pod-Like Supramicelles with Multicompartment Hydrophobic Cores Prepared by Self-Assembly of Modified Chitosan
Corresponding Author: Xuhong Guo
Nano-Micro Letters,
Vol. 8 No. 2 (2016), Article Number: 151-156
Abstract
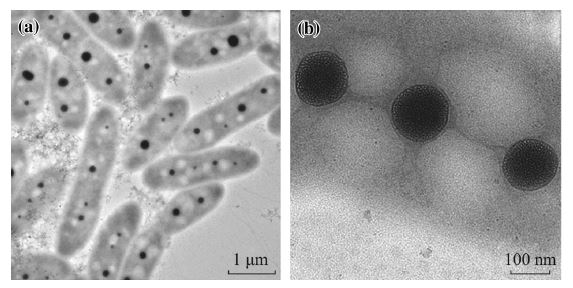
In this paper, pod-like supramicelles with multicompartment hydrophobic cores were prepared by self-assembly of amphiphilic N-phthaloylchitosan-g-poly(N-vinylcaprolactam) (PHCS-g-PNVCL) in aqueous medium. The employed biocompatible amphiphilic polymer was synthesized by grafting the carboxyl terminated poly(N-vinylcaprolactam) (PNVCL-COOH) chains onto N-phthaloylchitosan (PHCS) backbone. 1H NMR and FTIR results confirmed the molecular structure of the copolymers. The morphology of the supramicelles assembled by PHCS-g-PNVCL was revealed by means of TEM and polarized light microscope. In solution, the supramicelles were very stable as monitored by DLS and zeta potential measurements. Temperature and pH presented significant influences on the size and size distribution of the supramicelles. These supramicelles with multicompartment hydrophobic cores should be ideal biomimetic systems with promising applications in drug delivery.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- J.H. van Esch, Supramolecular chemistry: More than the sum of its parts. Nature 466(7303), 193–194 (2010). doi:10.1038/466193a
- T.P. Lodge, A. Rasdal, Z. Li, M.A. Hillmyer, Simultaneous, segregated storage of two agents in a multicompartment micelle. J. Am. Chem. Soc. 127(50), 17608–17609 (2005). doi:10.1021/ja056841t
- J. Zhu, R.C. Hayward, Wormlike micelles with microphase-separated cores from blends of amphiphilic AB and hydrophobic BC diblock copolymers. Macromolecules 41(21), 7794–7797 (2008). doi:10.1021/ma801783m
- A.O. Moughton, M.A. Hillmyer, T.P. Lodge, Multicompartment block polymer micelles. Macromolecules 45(1), 2–19 (2011). doi:10.1021/ma201865s
- J. Babinot, E. Renard, B. Le Droumaguet, J.M. Guigner, S. Mura, J. Nicolas, P. Couvreur, V. Langlois, Facile synthesis of multicompartment micelles based on biocompatible poly(3-hydroxyalkanoate). Macromol. Rapid Commun. 34(4), 362–368 (2013). doi:10.1002/marc.201200692
- J.N. Marsat, M. Heydenreich, E. Kleinpeter, H.V. Berlepsch, C. Bottcher, A. Laschewsky, Self-assembly into multicompartment micelles and selective solubilization by hydrophilic-lipophilic-fluorophilic block copolymers. Macromolecules 44(7), 2092–2105 (2011). doi:10.1021/ma200032j
- R. Weberskirch, J. Preuschen, H.W. Spiess, O. Nuyken, Design and synthesis of a two compartment micellar system based on the self-association behavior of poly(N-acylethyleneimine) end-capped with a fluorocarbon and a hydrocarbon chain. Macromol. Chem. Phys. 201(10), 995–1007 (2000). doi:10.1002/1521-3935(20000601)201:10<995:AID-MACP995>3.0.CO;2-T
- Z. Li, E. Kesselman, Y. Talmon, M.A. Hillmyer, T.P. Lodge, Multicompartment micelles from ABC miktoarm stars in water. Science 306, 98 (2004). doi:10.1126/science.1103350
- Z. Li, M.A. Hillmyer, T.P. Lodge, Morphologies of multicompartment micelles formed by ABC miktoarm star terpolymers. Langmuir 22(22), 9409–9417 (2006). doi:10.1021/la0620051
- Z. Li, M.A. Hillmyer, T.P. Lodge, Laterally nanostructured vesicles, polygonal bilayer sheets, and segmented wormlike micelles. Nano Lett. 6(6), 1245–1249 (2006). doi:10.1021/nl0608700
- H.V. Berlepsch, C. Bottcher, K. Skrabania, A. Laschewsky, Complex domain architecture of multicompartment micelles from a linear ABC triblock copolymer revealed by cryogenic electron tomography. Chem. Commun. 17, 2290–2292 (2009). doi:10.1039/b903658j
- A. Laschewsky, Polymerized micelles with compartments. Curr. Opin. Colloid Interf. Sci. 8(3), 274–281 (2003). doi:10.1016/S1359-0294(03)00049-9
- R. De Souza, P. Zahedi, C.J. Allen, M. Piquette-Miller, Biocompatibility of injectable chitosan–phospholipid implant systems. Biomaterials 30(23–24), 3818–3824 (2009). doi:10.1016/j.biomaterials.2009.04.003
- R. Hejazi, M. Amiji, Chitosan-based gastrointestinal delivery systems. J. Controll. Release 89(2), 151–165 (2003). doi:10.1016/S0168-3659(03)00126-3
- R. Muzzarelli, V. Baldassare, F. Conti, P. Ferrara, G. Biagini, G. Gazzanelli, V. Vasi, Biological activity of chitosan: Ultrastructural study. Biomaterials 9(3), 247–252 (1988). doi:10.1016/0142-9612(88)90092-0
- P. Mukhopadhyay, R. Mishra, D. Rana, P.P. Kundu, Strategies for effective oral insulin delivery with modified chitosan nanoparticles: A review. Prog. Polym. Sci. 37(11), 1457–1475 (2012). doi:10.1016/j.progpolymsci.2012.04.004
- G.M. Luz, L. Boesel, A.D. Campo, J.F. Mano, Micropatterning of bioactive glass nanoparticles on chitosan membranes for spatial controlled biomineralization. Langmuir 28(17), 6970–6977 (2012). doi:10.1021/la300667g
- W. Shi, D. Nie, G. Jin, W. Chen, L. Xia et al., BDNF blended chitosan scaffolds for human umbilical cord MSC transplants in traumatic brain injury therapy. Biomaterials 33(11), 3119–3126 (2012). doi:10.1016/j.biomaterials.2012.01.009
- N.Q. Tran, Y.K. Joung, E. Lih, K.D. Park, In situ forming and rutin-releasing chitosan hydrogels as injectable dressings for dermal wound healing. Biomacromolecules 12(8), 2872–2880 (2011). doi:10.1021/bm200326g
- P.T. Sudheesh Kumar, V.K. Lakshmanan, T.V. Anilkumar, C. Ramya, P. Reshmi, A.G. Unnikrishnan, S.V. Nair, R. Jayakumar, Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: in vitro and in vivo evaluation. ACS Appl. Mater. Inter. 4(5), 2618–2629 (2012). doi:10.1021/am300292v
- D. Deng, L. Qu, J. Zhang, Y. Ma, Y. Gu, Quaternary Zn–Ag–In–Se quantum dots for biomedical optical imaging of RGD-modified micelles. ACS Appl. Mater. Inter. 5(21), 10858–10865 (2013). doi:10.1021/am403050s
- L. Upadhyaya, J. Singh, V. Agarwal, R.P. Tewari, Biomedical applications of carboxymethyl chitosans. Carbohydr. Polym. 91(1), 452–466 (2013). doi:10.1016/j.carbpol.2012.07.076
- M.R. Kumar, R. Muzzarelli, C. Muzzarelli, H. Sashiwa, A.J. Domb, Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 104(11), 6017–6084 (2004). doi:10.1021/cr030441b
- K. Park, Controlled drug delivery systems: past forward and future back. J. Control. Release 190, 3–8 (2014). doi:10.1016/j.jconrel.2014.03.054
- H. Fessi, F. Puisieux, J.P. Devissaguet, N. Ammoury, S. Benita, Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 55(89), R1–R4 (1989). doi:10.1016/0378-5173(89)90281-0
- K. Kurita, H. Ikeda, Y. Yoshida, M. Shimojoh, M. Harata, Chemoselective protection of the amino groups of chitosan by controlled phthaloylation: facile preparation of a precursor useful for chemical modifications. Biomacromolecules 3(1), 1–4 (2002). doi:10.1021/bm0101163
- A. Blanazs, S.P. Armes, A.J. Ryan, Self-assembled block copolymer aggregates: from micelles to vesicles and their biological applications. Macromol. Rapid Commun. 30(4–5), 267–277 (2009). doi:10.1002/marc.200800713
- M. Polotsky, Y. Charlaganov, F.A. Xu, M. Leermakers, A.H.E. Daoud, T. Muller, O. Dotera, Borisov, Pearl-necklace structures in core-shell molecular brushes: Experiments, monte carlo simulations, and self-consistent field modeling. Macromolecules 41(11), 4020–4028 (2008). doi:10.1021/ma800125q
- A.V. Borisov, E.B. Zhulina, Amphiphilic graft copolymer in a selective solvent: Intramolecular structures and conformational transitions. Macromolecules 38(6), 2506–2514 (2005). doi:10.1021/ma047464s
- P. Kosovan, J. Kuldova, Z. Limpouchova, K. Prochazka, E.B. Zhulina, O.V. Borisov, Amphiphilic graft copolymers in selective solvents: Molecular dynamics simulations and scaling theory. Macromolecules 42(17), 6748–6760 (2009). doi:10.1021/ma900768p
- A. Anthony, R. Zana, Fluorescence investigation of the binding of pyrene to hydrophobic microdomains in aqueous solutions of polysoaps. Macromolecules 27(14), 3885–3891 (1994). doi:10.1021/ma00092a031
- K. Kalyanasundaram, J.K. Thomas, Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. JACS 99(7), 2039–2044 (1977). doi:10.1021/ja00449a004
- L.A. Fielding, M.J. Derry, V. Ladmiral, J. Rosselgong, A.M. Rodrigues, L. Ratcliffe, S. Sugihara, S.P. Armes, RAFT dispersion polymerization in non-polar solvents: Facile production of block copolymer spheres, worms and vesicles in n-alkanes. Chem. Sci. 4, 2081–2087 (2013). doi:10.1039/c3sc50305d
- R.J. Hunter, Zeta Potential in Colloid Science: Principles and Applications (Academic Press, CA, 1981)
References
J.H. van Esch, Supramolecular chemistry: More than the sum of its parts. Nature 466(7303), 193–194 (2010). doi:10.1038/466193a
T.P. Lodge, A. Rasdal, Z. Li, M.A. Hillmyer, Simultaneous, segregated storage of two agents in a multicompartment micelle. J. Am. Chem. Soc. 127(50), 17608–17609 (2005). doi:10.1021/ja056841t
J. Zhu, R.C. Hayward, Wormlike micelles with microphase-separated cores from blends of amphiphilic AB and hydrophobic BC diblock copolymers. Macromolecules 41(21), 7794–7797 (2008). doi:10.1021/ma801783m
A.O. Moughton, M.A. Hillmyer, T.P. Lodge, Multicompartment block polymer micelles. Macromolecules 45(1), 2–19 (2011). doi:10.1021/ma201865s
J. Babinot, E. Renard, B. Le Droumaguet, J.M. Guigner, S. Mura, J. Nicolas, P. Couvreur, V. Langlois, Facile synthesis of multicompartment micelles based on biocompatible poly(3-hydroxyalkanoate). Macromol. Rapid Commun. 34(4), 362–368 (2013). doi:10.1002/marc.201200692
J.N. Marsat, M. Heydenreich, E. Kleinpeter, H.V. Berlepsch, C. Bottcher, A. Laschewsky, Self-assembly into multicompartment micelles and selective solubilization by hydrophilic-lipophilic-fluorophilic block copolymers. Macromolecules 44(7), 2092–2105 (2011). doi:10.1021/ma200032j
R. Weberskirch, J. Preuschen, H.W. Spiess, O. Nuyken, Design and synthesis of a two compartment micellar system based on the self-association behavior of poly(N-acylethyleneimine) end-capped with a fluorocarbon and a hydrocarbon chain. Macromol. Chem. Phys. 201(10), 995–1007 (2000). doi:10.1002/1521-3935(20000601)201:10<995:AID-MACP995>3.0.CO;2-T
Z. Li, E. Kesselman, Y. Talmon, M.A. Hillmyer, T.P. Lodge, Multicompartment micelles from ABC miktoarm stars in water. Science 306, 98 (2004). doi:10.1126/science.1103350
Z. Li, M.A. Hillmyer, T.P. Lodge, Morphologies of multicompartment micelles formed by ABC miktoarm star terpolymers. Langmuir 22(22), 9409–9417 (2006). doi:10.1021/la0620051
Z. Li, M.A. Hillmyer, T.P. Lodge, Laterally nanostructured vesicles, polygonal bilayer sheets, and segmented wormlike micelles. Nano Lett. 6(6), 1245–1249 (2006). doi:10.1021/nl0608700
H.V. Berlepsch, C. Bottcher, K. Skrabania, A. Laschewsky, Complex domain architecture of multicompartment micelles from a linear ABC triblock copolymer revealed by cryogenic electron tomography. Chem. Commun. 17, 2290–2292 (2009). doi:10.1039/b903658j
A. Laschewsky, Polymerized micelles with compartments. Curr. Opin. Colloid Interf. Sci. 8(3), 274–281 (2003). doi:10.1016/S1359-0294(03)00049-9
R. De Souza, P. Zahedi, C.J. Allen, M. Piquette-Miller, Biocompatibility of injectable chitosan–phospholipid implant systems. Biomaterials 30(23–24), 3818–3824 (2009). doi:10.1016/j.biomaterials.2009.04.003
R. Hejazi, M. Amiji, Chitosan-based gastrointestinal delivery systems. J. Controll. Release 89(2), 151–165 (2003). doi:10.1016/S0168-3659(03)00126-3
R. Muzzarelli, V. Baldassare, F. Conti, P. Ferrara, G. Biagini, G. Gazzanelli, V. Vasi, Biological activity of chitosan: Ultrastructural study. Biomaterials 9(3), 247–252 (1988). doi:10.1016/0142-9612(88)90092-0
P. Mukhopadhyay, R. Mishra, D. Rana, P.P. Kundu, Strategies for effective oral insulin delivery with modified chitosan nanoparticles: A review. Prog. Polym. Sci. 37(11), 1457–1475 (2012). doi:10.1016/j.progpolymsci.2012.04.004
G.M. Luz, L. Boesel, A.D. Campo, J.F. Mano, Micropatterning of bioactive glass nanoparticles on chitosan membranes for spatial controlled biomineralization. Langmuir 28(17), 6970–6977 (2012). doi:10.1021/la300667g
W. Shi, D. Nie, G. Jin, W. Chen, L. Xia et al., BDNF blended chitosan scaffolds for human umbilical cord MSC transplants in traumatic brain injury therapy. Biomaterials 33(11), 3119–3126 (2012). doi:10.1016/j.biomaterials.2012.01.009
N.Q. Tran, Y.K. Joung, E. Lih, K.D. Park, In situ forming and rutin-releasing chitosan hydrogels as injectable dressings for dermal wound healing. Biomacromolecules 12(8), 2872–2880 (2011). doi:10.1021/bm200326g
P.T. Sudheesh Kumar, V.K. Lakshmanan, T.V. Anilkumar, C. Ramya, P. Reshmi, A.G. Unnikrishnan, S.V. Nair, R. Jayakumar, Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: in vitro and in vivo evaluation. ACS Appl. Mater. Inter. 4(5), 2618–2629 (2012). doi:10.1021/am300292v
D. Deng, L. Qu, J. Zhang, Y. Ma, Y. Gu, Quaternary Zn–Ag–In–Se quantum dots for biomedical optical imaging of RGD-modified micelles. ACS Appl. Mater. Inter. 5(21), 10858–10865 (2013). doi:10.1021/am403050s
L. Upadhyaya, J. Singh, V. Agarwal, R.P. Tewari, Biomedical applications of carboxymethyl chitosans. Carbohydr. Polym. 91(1), 452–466 (2013). doi:10.1016/j.carbpol.2012.07.076
M.R. Kumar, R. Muzzarelli, C. Muzzarelli, H. Sashiwa, A.J. Domb, Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 104(11), 6017–6084 (2004). doi:10.1021/cr030441b
K. Park, Controlled drug delivery systems: past forward and future back. J. Control. Release 190, 3–8 (2014). doi:10.1016/j.jconrel.2014.03.054
H. Fessi, F. Puisieux, J.P. Devissaguet, N. Ammoury, S. Benita, Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 55(89), R1–R4 (1989). doi:10.1016/0378-5173(89)90281-0
K. Kurita, H. Ikeda, Y. Yoshida, M. Shimojoh, M. Harata, Chemoselective protection of the amino groups of chitosan by controlled phthaloylation: facile preparation of a precursor useful for chemical modifications. Biomacromolecules 3(1), 1–4 (2002). doi:10.1021/bm0101163
A. Blanazs, S.P. Armes, A.J. Ryan, Self-assembled block copolymer aggregates: from micelles to vesicles and their biological applications. Macromol. Rapid Commun. 30(4–5), 267–277 (2009). doi:10.1002/marc.200800713
M. Polotsky, Y. Charlaganov, F.A. Xu, M. Leermakers, A.H.E. Daoud, T. Muller, O. Dotera, Borisov, Pearl-necklace structures in core-shell molecular brushes: Experiments, monte carlo simulations, and self-consistent field modeling. Macromolecules 41(11), 4020–4028 (2008). doi:10.1021/ma800125q
A.V. Borisov, E.B. Zhulina, Amphiphilic graft copolymer in a selective solvent: Intramolecular structures and conformational transitions. Macromolecules 38(6), 2506–2514 (2005). doi:10.1021/ma047464s
P. Kosovan, J. Kuldova, Z. Limpouchova, K. Prochazka, E.B. Zhulina, O.V. Borisov, Amphiphilic graft copolymers in selective solvents: Molecular dynamics simulations and scaling theory. Macromolecules 42(17), 6748–6760 (2009). doi:10.1021/ma900768p
A. Anthony, R. Zana, Fluorescence investigation of the binding of pyrene to hydrophobic microdomains in aqueous solutions of polysoaps. Macromolecules 27(14), 3885–3891 (1994). doi:10.1021/ma00092a031
K. Kalyanasundaram, J.K. Thomas, Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. JACS 99(7), 2039–2044 (1977). doi:10.1021/ja00449a004
L.A. Fielding, M.J. Derry, V. Ladmiral, J. Rosselgong, A.M. Rodrigues, L. Ratcliffe, S. Sugihara, S.P. Armes, RAFT dispersion polymerization in non-polar solvents: Facile production of block copolymer spheres, worms and vesicles in n-alkanes. Chem. Sci. 4, 2081–2087 (2013). doi:10.1039/c3sc50305d
R.J. Hunter, Zeta Potential in Colloid Science: Principles and Applications (Academic Press, CA, 1981)