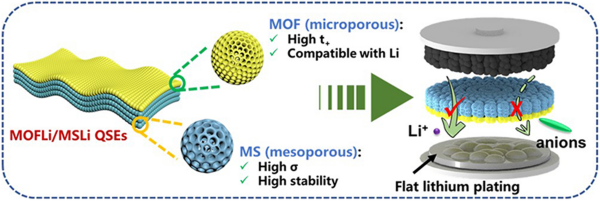
Janus Quasi-Solid Electrolyte Membranes with Asymmetric Porous Structure for High-Performance Lithium-Metal Batteries
Corresponding Author: Hao Bin Wu
Nano-Micro Letters,
Vol. 16 (2024), Article Number: 114
Abstract
Quasi-solid electrolytes (QSEs) based on nanoporous materials are promising candidates to construct high-performance Li-metal batteries (LMBs). However, simultaneously boosting the ionic conductivity (σ) and lithium-ion transference number (t+) of liquid electrolyte confined in porous matrix remains challenging. Herein, we report a novel Janus MOFLi/MSLi QSEs with asymmetric porous structure to inherit the benefits of both mesoporous and microporous hosts. This Janus QSE composed of mesoporous silica and microporous MOF exhibits a neat Li+ conductivity of 1.5 × 10–4 S cm−1 with t+ of 0.71. A partially de-solvated structure and preference distribution of Li+ near the Lewis base O atoms were depicted by MD simulations. Meanwhile, the nanoporous structure enabled efficient ion flux regulation, promoting the homogenous deposition of Li+. When incorporated in Li||Cu cells, the MOFLi/MSLi QSEs demonstrated a high Coulombic efficiency of 98.1%, surpassing that of liquid electrolytes (96.3%). Additionally, NCM 622||Li batteries equipped with MOFLi/MSLi QSEs exhibited promising rate performance and could operate stably for over 200 cycles at 1 C. These results highlight the potential of Janus MOFLi/MSLi QSEs as promising candidates for next-generation LMBs.
Highlights:
1 Janus quasi-solid electrolyte membranes with asymmetric porous structure were constructed, showing a high σLi+ of 1.5 × 10-4 S cm-1 and a high t+ of 0.71.
2 The solvation structures and ion transport dynamics in nanopores have been deciphered, manifesting a concentrated electrolyte-like structure and regulated transport behaviors.
3 Quasi-solid NCM 622||Li cells have been demonstrated to stably cycle for 200 cycles at 1 C, and pouch cell has shown high tolerance for abuse.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- H. Furukawa, K.E. Cordova, M. O’Keeffe, O.M. Yaghi, The chemistry and applications of metal–organic frameworks. Science 341, 1230444 (2013). https://doi.org/10.1126/science.1230444
- J.L.C. Rowsell, O.M. Yaghi, Metal–organic frameworks: a new class of porous materials. Microporous Mesoporous Mater. 73, 3–14 (2004). https://doi.org/10.1016/j.micromeso.2004.03.034
- S.-Y. Ding, W. Wang, Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 42, 548–568 (2013). https://doi.org/10.1039/C2CS35072F
- X. Feng, X. Ding, D. Jiang, Covalent organic frameworks. Chem. Soc. Rev. 41, 6010–6022 (2012). https://doi.org/10.1039/c2cs35157a
- K. Geng, T. He, R. Liu, S. Dalapati, K.T. Tan et al., Covalent organic frameworks: design, synthesis, and functions. Chem. Rev. 120, 8814–8933 (2020). https://doi.org/10.1021/acs.chemrev.9b00550
- U. Olsbye, S. Svelle, M. Bjørgen, P. Beato, T.V. Janssens et al., Conversion of methanol to hydrocarbons: How zeolite cavity and pore size controls product selectivity. Angew. Chem. Int. Ed. 51, 5810–5831 (2012). https://doi.org/10.1002/anie.201103657
- Z. Chang, H. Yang, X. Zhu, P. He, H. Zhou, A stable quasi-solid electrolyte improves the safe operation of highly efficient lithium-metal pouch cells in harsh environments. Nat. Commun. 13, 1510 (2022). https://doi.org/10.1038/s41467-022-29118-6
- L. Shen, H.B. Wu, F. Liu, J.L. Brosmer, G. Shen et al., Creating lithium-ion electrolytes with biomimetic ionic channels in metal-organic frameworks. Adv. Mater. 30, e1707476 (2018). https://doi.org/10.1002/adma.201707476
- B.M. Wiers, M.-L. Foo, N.P. Balsara, J.R. Long, A solid lithium electrolyte via addition of lithium isopropoxide to a metal–organic framework with open metal sites. J. Am. Chem. Soc. 133, 14522–14525 (2011). https://doi.org/10.1021/ja205827z
- S. Bai, X. Liu, K. Zhu, S. Wu, H. Zhou, Metal–organic framework-based separator for lithium–sulfur batteries. Nat. Energy 1, 16094 (2016). https://doi.org/10.1038/nenergy.2016.94
- H. Chen, H. Tu, C. Hu, Y. Liu, D. Dong et al., Cationic covalent organic framework nanosheets for fast Li-ion conduction. J. Am. Chem. Soc. 140, 896–899 (2018). https://doi.org/10.1021/jacs.7b12292
- X. Li, Q. Hou, W. Huang, H.-S. Xu, X. Wang et al., Solution-processable covalent organic framework electrolytes for all-solid-state Li–organic batteries. ACS Energy Lett. 5, 3498–3506 (2020). https://doi.org/10.1021/acsenergylett.0c01889
- X. Chi, M. Li, J. Di, P. Bai, L. Song et al., A highly stable and flexible zeolite electrolyte solid-state Li-air battery. Nature 592, 551–557 (2021). https://doi.org/10.1038/s41586-021-03410-9
- Y. Xu, L. Gao, Q. Liu, Q. Liu, Z. Chen et al., Segmental molecular dynamics boosts Li-ion conduction in metal-organic solid electrolytes for Li-metal batteries. Energy Storage Mater. 54, 854–862 (2023). https://doi.org/10.1016/j.ensm.2022.11.029
- H. Yang, B. Liu, J. Bright, S. Kasani, J. Yang et al., A single-ion conducting UiO-66 metal–organic framework electrolyte for all-solid-state lithium batteries. ACS Appl. Energy Mater. 3, 4007–4013 (2020). https://doi.org/10.1021/acsaem.0c00410
- F. Zhu, H. Bao, X. Wu, Y. Tao, C. Qin et al., High-performance metal-organic framework-based single ion conducting solid-state electrolytes for low-temperature lithium metal batteries. ACS Appl. Mater. Interfaces 11, 43206–43213 (2019). https://doi.org/10.1021/acsami.9b15374
- Z. Chang, Y. Qiao, H. Deng, H. Yang, P. He et al., A liquid electrolyte with de-solvated lithium ions for lithium-metal battery. Joule 4, 1776–1789 (2020). https://doi.org/10.1016/j.joule.2020.06.011
- Z. Chang, Y. Qiao, H. Yang, X. Cao, X. Zhu et al., Sustainable lithium-metal battery achieved by a safe electrolyte based on recyclable and low-cost molecular sieve. Angew. Chem. Int. Ed. 60, 15572–15581 (2021). https://doi.org/10.1002/anie.202104124
- Z. Chang, H. Yang, Y. Qiao, X. Zhu, P. He et al., Tailoring the solvation sheath of cations by constructing electrode front-faces for rechargeable batteries. Adv. Mater. 34, e2201339 (2022). https://doi.org/10.1002/adma.202201339
- H.J.S. Sand III., On the concentration at the electrodes in a solution, with special reference to the liberation of hydrogen by electrolysis of a mixture of copper sulphate and sulphuric acid. Lond. Edinb Dublin Philos. Mag. J. Sci. 1, 45–79 (1901). https://doi.org/10.1080/14786440109462590
- P. Dong, X. Zhang, W. Hiscox, J. Liu, J. Zamora et al., Toward high-performance metal-organic-framework-based quasi-solid-state electrolytes: tunable structures and electrochemical properties. Adv. Mater. 35, e2211841 (2023). https://doi.org/10.1002/adma.202211841
- M.L. Aubrey, R. Ameloot, B.M. Wiers, J.R. Long, Metal–organic frameworks as solid magnesium electrolytes. Energy Environ. Sci. 7, 667–671 (2014). https://doi.org/10.1039/C3EE43143F
- W. He, D. Li, S. Guo, Y. Xiao, W. Gong et al., Redistribution of electronic density in channels of metal–Organic frameworks for high-performance quasi-solid lithium metal batteries. Energy Storage Mater. 47, 271–278 (2022). https://doi.org/10.1016/j.ensm.2022.02.003
- T. Hou, W. Xu, X. Pei, L. Jiang, O.M. Yaghi et al., Ionic conduction mechanism and design of metal-organic framework based quasi-solid-state electrolytes. J. Am. Chem. Soc. 144, 13446–13450 (2022). https://doi.org/10.1021/jacs.2c03710
- Z. Miao, F. Zhang, H. Zhao, M. Du, H. Li et al., Tailoring local electrolyte solvation structure via a mesoporous molecular sieve for dendrite-free zinc batteries. Adv. Funct. Mater. 32, 2111635 (2022). https://doi.org/10.1002/adfm.202111635
- L. Han, Z. Wang, D. Kong, L. Yang, K. Yang et al., An ordered mesoporous silica framework based electrolyte with nanowetted interfaces for solid-state lithium batteries. J. Mater. Chem. A 6, 21280–21286 (2018). https://doi.org/10.1039/C8TA08875F
- K. Wang, C. Li, Y. Liang, T. Han, H. Huang et al., Rational construction of defects in a metal–organic framework for highly efficient adsorption and separation of dyes. Chem. Eng. J. 289, 486–493 (2016). https://doi.org/10.1016/j.cej.2016.01.019
- Z. Li, Q. Liu, L. Gao, Y. Xu, X. Kong et al., Quasi-solid electrolyte membranes with percolated metal–organic frameworks for practical lithium-metal batteries. J. Energy Chem. 52, 354–360 (2021). https://doi.org/10.1016/j.jechem.2020.04.013
- Y. Xu, L. Gao, L. Shen, Q. Liu, Y. Zhu et al., Ion-transport-rectifying layer enables Li-metal batteries with high energy density. Matter 3, 1685–1700 (2020). https://doi.org/10.1016/j.matt.2020.08.011
- G. Wang, J. Gao, Y. Fu, Z. Ren, J. Huang et al., Implantable composite fibres with Self-supplied H2O2 for localized chemodynamic therapy. Chem. Eng. J. 388, 124211 (2020). https://doi.org/10.1016/j.cej.2020.124211
- L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M.H. Nilsen et al., Disclosing the complex structure of UiO-66 metal organic framework: a synergic combination of experiment and theory. Chem. Mater. 23, 1700–1718 (2011). https://doi.org/10.1021/cm1022882
- G.C. Shearer, S. Chavan, S. Bordiga, S. Svelle, U. Olsbye et al., Defect engineering: tuning the porosity and composition of the metal–organic framework UiO-66 via modulated synthesis. Chem. Mater. 28, 3749–3761 (2016). https://doi.org/10.1021/acs.chemmater.6b00602
- S. Mohebbi, M. Shariatipour, B. Shafie, M.M. Amini, Encapsulation of tamoxifen citrate in functionalized mesoporous silica and investigation of its release. J. Drug Deliv. Sci. Technol. 62, 102406 (2021). https://doi.org/10.1016/j.jddst.2021.102406
- M. Taddei, When defects turn into virtues: the curious e of zirconium-based metal-organic frameworks. Coord. Chem. Rev. 343, 1–24 (2017). https://doi.org/10.1016/j.ccr.2017.04.010
- L. Cai, H. Ying, P. Huang, Z. Zhang, H. Tan et al., In-situ grown Ti3C2T @CoSe2 heterostructure as trapping-electrocatalyst for accelerating polysulfides conversion in lithium-sulfur battery. Chem. Eng. J. 474, 145862 (2023). https://doi.org/10.1016/j.cej.2023.145862
- L. Liu, Z. Chen, J. Wang, D. Zhang, Y. Zhu et al., Imaging defects and their evolution in a metal-organic framework at sub-unit-cell resolution. Nat. Chem. 11, 622–628 (2019). https://doi.org/10.1038/s41557-019-0263-4
- Q. Han, L. Cai, P. Huang, S. Liu, C. He et al., Fast ionic conducting hydroxyapatite solid electrolyte interphase enables ultra-stable zinc metal anodes. ACS Appl. Mater. Interfaces 15, 48316–48325 (2023). https://doi.org/10.1021/acsami.3c11649
- Z. Wang, W. Huang, J. Hua, Y. Wang, H. Yi et al., An anionic-MOF-based bifunctional separator for regulating lithium deposition and suppressing polysulfides shuttle in Li–S batteries. Small Meth. 4, 2000082 (2020). https://doi.org/10.1002/smtd.202000082
- Y. Sun, T. Yang, H. Ji, J. Zhou, Z. Wang et al., Boosting the optimization of lithium metal batteries by molecular dynamics simulations: a perspective. Adv. Energy Mater. 10, 2002373 (2020). https://doi.org/10.1002/aenm.202002373
- S. Bai, Y. Sun, J. Yi, Y. He, Y. Qiao et al., High-power Li-metal anode enabled by metal-organic framework modified electrolyte. Joule 2, 2117–2132 (2018). https://doi.org/10.1016/j.joule.2018.07.010
- S. Yuan, J.L. Bao, J. Wei, Y. Xia, D.G. Truhlar et al., A versatile single-ion electrolyte with a Grotthuss-like Li conduction mechanism for dendrite-free Li metal batteries. Energy Environ. Sci. 12, 2741–2750 (2019). https://doi.org/10.1039/C9EE01473J
- M.F. Döpke, J. Lützenkirchen, O.A. Moultos, B. Siboulet, J.-F. Dufrêche et al., Preferential adsorption in mixed electrolytes confined by charged amorphous silica. J. Phys. Chem. C 123, 16711–16720 (2019). https://doi.org/10.1021/acs.jpcc.9b02975
- K. Qian, S. Seifert, R.E. Winans, T. Li, Understanding solvation behavior of the saturated electrolytes with small/wide-angle X-ray scattering and Raman spectroscopy. Energy Fuels 35, 19849–19855 (2021). https://doi.org/10.1021/acs.energyfuels.1c03328
- L. Cao, D. Li, T. Deng, Q. Li, C. Wang, Hydrophobic organic-electrolyte-protected zinc anodes for aqueous zinc batteries. Angew. Chem. Int. Ed. 59, 19292–19296 (2020). https://doi.org/10.1002/anie.202008634
- H. Gan, J. Wu, F. Zhang, R. Li, H. Liu, Uniform Zn2+ distribution and deposition regulated by ultrathin hydroxyl-rich silica ion sieve in zinc metal anodes. Energy Storage Mater. 55, 264–271 (2023). https://doi.org/10.1016/j.ensm.2022.11.044
- X.-X. Wang, X.-W. Chi, M.-L. Li, D.-H. Guan, C.-L. Miao et al., Metal–organic frameworks derived electrolytes build multiple wetting interfaces for integrated solid-state lithium–oxygen battery. Adv. Funct. Mater. 32, 2113235 (2022). https://doi.org/10.1002/adfm.202113235
- B.G. Lee, Y.J. Park, Enhanced electrochemical performance of lithia/Li2RuO3 cathode by adding tris(trimethylsilyl)borate as electrolyte additive. Sci. Rep. 10, 13498 (2020). https://doi.org/10.1038/s41598-020-70333-2
- H. Ma, D. Hwang, Y.J. Ahn, M.-Y. Lee, S. Kim et al., In situ interfacial tuning to obtain high-performance nickel-rich cathodes in lithium metal batteries. ACS Appl. Mater. Interfaces 12, 29365–29375 (2020). https://doi.org/10.1021/acsami.0c06830
- H.Q. Pham, M. Mirolo, M. Tarik, M. El Kazzi, S. Trabesinger, Multifunctional electrolyte additive for improved interfacial stability in Ni-rich layered oxide full-cells. Energy Storage Mater. 33, 216–229 (2020). https://doi.org/10.1016/j.ensm.2020.08.026
References
H. Furukawa, K.E. Cordova, M. O’Keeffe, O.M. Yaghi, The chemistry and applications of metal–organic frameworks. Science 341, 1230444 (2013). https://doi.org/10.1126/science.1230444
J.L.C. Rowsell, O.M. Yaghi, Metal–organic frameworks: a new class of porous materials. Microporous Mesoporous Mater. 73, 3–14 (2004). https://doi.org/10.1016/j.micromeso.2004.03.034
S.-Y. Ding, W. Wang, Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 42, 548–568 (2013). https://doi.org/10.1039/C2CS35072F
X. Feng, X. Ding, D. Jiang, Covalent organic frameworks. Chem. Soc. Rev. 41, 6010–6022 (2012). https://doi.org/10.1039/c2cs35157a
K. Geng, T. He, R. Liu, S. Dalapati, K.T. Tan et al., Covalent organic frameworks: design, synthesis, and functions. Chem. Rev. 120, 8814–8933 (2020). https://doi.org/10.1021/acs.chemrev.9b00550
U. Olsbye, S. Svelle, M. Bjørgen, P. Beato, T.V. Janssens et al., Conversion of methanol to hydrocarbons: How zeolite cavity and pore size controls product selectivity. Angew. Chem. Int. Ed. 51, 5810–5831 (2012). https://doi.org/10.1002/anie.201103657
Z. Chang, H. Yang, X. Zhu, P. He, H. Zhou, A stable quasi-solid electrolyte improves the safe operation of highly efficient lithium-metal pouch cells in harsh environments. Nat. Commun. 13, 1510 (2022). https://doi.org/10.1038/s41467-022-29118-6
L. Shen, H.B. Wu, F. Liu, J.L. Brosmer, G. Shen et al., Creating lithium-ion electrolytes with biomimetic ionic channels in metal-organic frameworks. Adv. Mater. 30, e1707476 (2018). https://doi.org/10.1002/adma.201707476
B.M. Wiers, M.-L. Foo, N.P. Balsara, J.R. Long, A solid lithium electrolyte via addition of lithium isopropoxide to a metal–organic framework with open metal sites. J. Am. Chem. Soc. 133, 14522–14525 (2011). https://doi.org/10.1021/ja205827z
S. Bai, X. Liu, K. Zhu, S. Wu, H. Zhou, Metal–organic framework-based separator for lithium–sulfur batteries. Nat. Energy 1, 16094 (2016). https://doi.org/10.1038/nenergy.2016.94
H. Chen, H. Tu, C. Hu, Y. Liu, D. Dong et al., Cationic covalent organic framework nanosheets for fast Li-ion conduction. J. Am. Chem. Soc. 140, 896–899 (2018). https://doi.org/10.1021/jacs.7b12292
X. Li, Q. Hou, W. Huang, H.-S. Xu, X. Wang et al., Solution-processable covalent organic framework electrolytes for all-solid-state Li–organic batteries. ACS Energy Lett. 5, 3498–3506 (2020). https://doi.org/10.1021/acsenergylett.0c01889
X. Chi, M. Li, J. Di, P. Bai, L. Song et al., A highly stable and flexible zeolite electrolyte solid-state Li-air battery. Nature 592, 551–557 (2021). https://doi.org/10.1038/s41586-021-03410-9
Y. Xu, L. Gao, Q. Liu, Q. Liu, Z. Chen et al., Segmental molecular dynamics boosts Li-ion conduction in metal-organic solid electrolytes for Li-metal batteries. Energy Storage Mater. 54, 854–862 (2023). https://doi.org/10.1016/j.ensm.2022.11.029
H. Yang, B. Liu, J. Bright, S. Kasani, J. Yang et al., A single-ion conducting UiO-66 metal–organic framework electrolyte for all-solid-state lithium batteries. ACS Appl. Energy Mater. 3, 4007–4013 (2020). https://doi.org/10.1021/acsaem.0c00410
F. Zhu, H. Bao, X. Wu, Y. Tao, C. Qin et al., High-performance metal-organic framework-based single ion conducting solid-state electrolytes for low-temperature lithium metal batteries. ACS Appl. Mater. Interfaces 11, 43206–43213 (2019). https://doi.org/10.1021/acsami.9b15374
Z. Chang, Y. Qiao, H. Deng, H. Yang, P. He et al., A liquid electrolyte with de-solvated lithium ions for lithium-metal battery. Joule 4, 1776–1789 (2020). https://doi.org/10.1016/j.joule.2020.06.011
Z. Chang, Y. Qiao, H. Yang, X. Cao, X. Zhu et al., Sustainable lithium-metal battery achieved by a safe electrolyte based on recyclable and low-cost molecular sieve. Angew. Chem. Int. Ed. 60, 15572–15581 (2021). https://doi.org/10.1002/anie.202104124
Z. Chang, H. Yang, Y. Qiao, X. Zhu, P. He et al., Tailoring the solvation sheath of cations by constructing electrode front-faces for rechargeable batteries. Adv. Mater. 34, e2201339 (2022). https://doi.org/10.1002/adma.202201339
H.J.S. Sand III., On the concentration at the electrodes in a solution, with special reference to the liberation of hydrogen by electrolysis of a mixture of copper sulphate and sulphuric acid. Lond. Edinb Dublin Philos. Mag. J. Sci. 1, 45–79 (1901). https://doi.org/10.1080/14786440109462590
P. Dong, X. Zhang, W. Hiscox, J. Liu, J. Zamora et al., Toward high-performance metal-organic-framework-based quasi-solid-state electrolytes: tunable structures and electrochemical properties. Adv. Mater. 35, e2211841 (2023). https://doi.org/10.1002/adma.202211841
M.L. Aubrey, R. Ameloot, B.M. Wiers, J.R. Long, Metal–organic frameworks as solid magnesium electrolytes. Energy Environ. Sci. 7, 667–671 (2014). https://doi.org/10.1039/C3EE43143F
W. He, D. Li, S. Guo, Y. Xiao, W. Gong et al., Redistribution of electronic density in channels of metal–Organic frameworks for high-performance quasi-solid lithium metal batteries. Energy Storage Mater. 47, 271–278 (2022). https://doi.org/10.1016/j.ensm.2022.02.003
T. Hou, W. Xu, X. Pei, L. Jiang, O.M. Yaghi et al., Ionic conduction mechanism and design of metal-organic framework based quasi-solid-state electrolytes. J. Am. Chem. Soc. 144, 13446–13450 (2022). https://doi.org/10.1021/jacs.2c03710
Z. Miao, F. Zhang, H. Zhao, M. Du, H. Li et al., Tailoring local electrolyte solvation structure via a mesoporous molecular sieve for dendrite-free zinc batteries. Adv. Funct. Mater. 32, 2111635 (2022). https://doi.org/10.1002/adfm.202111635
L. Han, Z. Wang, D. Kong, L. Yang, K. Yang et al., An ordered mesoporous silica framework based electrolyte with nanowetted interfaces for solid-state lithium batteries. J. Mater. Chem. A 6, 21280–21286 (2018). https://doi.org/10.1039/C8TA08875F
K. Wang, C. Li, Y. Liang, T. Han, H. Huang et al., Rational construction of defects in a metal–organic framework for highly efficient adsorption and separation of dyes. Chem. Eng. J. 289, 486–493 (2016). https://doi.org/10.1016/j.cej.2016.01.019
Z. Li, Q. Liu, L. Gao, Y. Xu, X. Kong et al., Quasi-solid electrolyte membranes with percolated metal–organic frameworks for practical lithium-metal batteries. J. Energy Chem. 52, 354–360 (2021). https://doi.org/10.1016/j.jechem.2020.04.013
Y. Xu, L. Gao, L. Shen, Q. Liu, Y. Zhu et al., Ion-transport-rectifying layer enables Li-metal batteries with high energy density. Matter 3, 1685–1700 (2020). https://doi.org/10.1016/j.matt.2020.08.011
G. Wang, J. Gao, Y. Fu, Z. Ren, J. Huang et al., Implantable composite fibres with Self-supplied H2O2 for localized chemodynamic therapy. Chem. Eng. J. 388, 124211 (2020). https://doi.org/10.1016/j.cej.2020.124211
L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M.H. Nilsen et al., Disclosing the complex structure of UiO-66 metal organic framework: a synergic combination of experiment and theory. Chem. Mater. 23, 1700–1718 (2011). https://doi.org/10.1021/cm1022882
G.C. Shearer, S. Chavan, S. Bordiga, S. Svelle, U. Olsbye et al., Defect engineering: tuning the porosity and composition of the metal–organic framework UiO-66 via modulated synthesis. Chem. Mater. 28, 3749–3761 (2016). https://doi.org/10.1021/acs.chemmater.6b00602
S. Mohebbi, M. Shariatipour, B. Shafie, M.M. Amini, Encapsulation of tamoxifen citrate in functionalized mesoporous silica and investigation of its release. J. Drug Deliv. Sci. Technol. 62, 102406 (2021). https://doi.org/10.1016/j.jddst.2021.102406
M. Taddei, When defects turn into virtues: the curious e of zirconium-based metal-organic frameworks. Coord. Chem. Rev. 343, 1–24 (2017). https://doi.org/10.1016/j.ccr.2017.04.010
L. Cai, H. Ying, P. Huang, Z. Zhang, H. Tan et al., In-situ grown Ti3C2T @CoSe2 heterostructure as trapping-electrocatalyst for accelerating polysulfides conversion in lithium-sulfur battery. Chem. Eng. J. 474, 145862 (2023). https://doi.org/10.1016/j.cej.2023.145862
L. Liu, Z. Chen, J. Wang, D. Zhang, Y. Zhu et al., Imaging defects and their evolution in a metal-organic framework at sub-unit-cell resolution. Nat. Chem. 11, 622–628 (2019). https://doi.org/10.1038/s41557-019-0263-4
Q. Han, L. Cai, P. Huang, S. Liu, C. He et al., Fast ionic conducting hydroxyapatite solid electrolyte interphase enables ultra-stable zinc metal anodes. ACS Appl. Mater. Interfaces 15, 48316–48325 (2023). https://doi.org/10.1021/acsami.3c11649
Z. Wang, W. Huang, J. Hua, Y. Wang, H. Yi et al., An anionic-MOF-based bifunctional separator for regulating lithium deposition and suppressing polysulfides shuttle in Li–S batteries. Small Meth. 4, 2000082 (2020). https://doi.org/10.1002/smtd.202000082
Y. Sun, T. Yang, H. Ji, J. Zhou, Z. Wang et al., Boosting the optimization of lithium metal batteries by molecular dynamics simulations: a perspective. Adv. Energy Mater. 10, 2002373 (2020). https://doi.org/10.1002/aenm.202002373
S. Bai, Y. Sun, J. Yi, Y. He, Y. Qiao et al., High-power Li-metal anode enabled by metal-organic framework modified electrolyte. Joule 2, 2117–2132 (2018). https://doi.org/10.1016/j.joule.2018.07.010
S. Yuan, J.L. Bao, J. Wei, Y. Xia, D.G. Truhlar et al., A versatile single-ion electrolyte with a Grotthuss-like Li conduction mechanism for dendrite-free Li metal batteries. Energy Environ. Sci. 12, 2741–2750 (2019). https://doi.org/10.1039/C9EE01473J
M.F. Döpke, J. Lützenkirchen, O.A. Moultos, B. Siboulet, J.-F. Dufrêche et al., Preferential adsorption in mixed electrolytes confined by charged amorphous silica. J. Phys. Chem. C 123, 16711–16720 (2019). https://doi.org/10.1021/acs.jpcc.9b02975
K. Qian, S. Seifert, R.E. Winans, T. Li, Understanding solvation behavior of the saturated electrolytes with small/wide-angle X-ray scattering and Raman spectroscopy. Energy Fuels 35, 19849–19855 (2021). https://doi.org/10.1021/acs.energyfuels.1c03328
L. Cao, D. Li, T. Deng, Q. Li, C. Wang, Hydrophobic organic-electrolyte-protected zinc anodes for aqueous zinc batteries. Angew. Chem. Int. Ed. 59, 19292–19296 (2020). https://doi.org/10.1002/anie.202008634
H. Gan, J. Wu, F. Zhang, R. Li, H. Liu, Uniform Zn2+ distribution and deposition regulated by ultrathin hydroxyl-rich silica ion sieve in zinc metal anodes. Energy Storage Mater. 55, 264–271 (2023). https://doi.org/10.1016/j.ensm.2022.11.044
X.-X. Wang, X.-W. Chi, M.-L. Li, D.-H. Guan, C.-L. Miao et al., Metal–organic frameworks derived electrolytes build multiple wetting interfaces for integrated solid-state lithium–oxygen battery. Adv. Funct. Mater. 32, 2113235 (2022). https://doi.org/10.1002/adfm.202113235
B.G. Lee, Y.J. Park, Enhanced electrochemical performance of lithia/Li2RuO3 cathode by adding tris(trimethylsilyl)borate as electrolyte additive. Sci. Rep. 10, 13498 (2020). https://doi.org/10.1038/s41598-020-70333-2
H. Ma, D. Hwang, Y.J. Ahn, M.-Y. Lee, S. Kim et al., In situ interfacial tuning to obtain high-performance nickel-rich cathodes in lithium metal batteries. ACS Appl. Mater. Interfaces 12, 29365–29375 (2020). https://doi.org/10.1021/acsami.0c06830
H.Q. Pham, M. Mirolo, M. Tarik, M. El Kazzi, S. Trabesinger, Multifunctional electrolyte additive for improved interfacial stability in Ni-rich layered oxide full-cells. Energy Storage Mater. 33, 216–229 (2020). https://doi.org/10.1016/j.ensm.2020.08.026