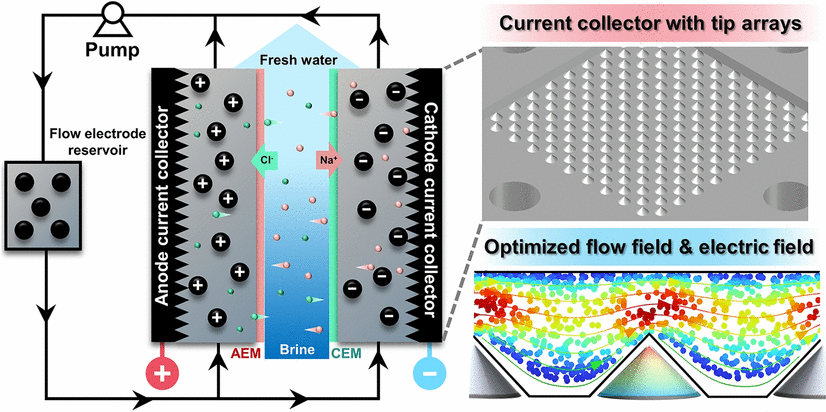
Locally Enhanced Flow and Electric Fields Through a Tip Effect for Efficient Flow-Electrode Capacitive Deionization
Corresponding Author: Libo Deng
Nano-Micro Letters,
Vol. 17 (2025), Article Number: 26
Abstract
Low-electrode capacitive deionization (FCDI) is an emerging desalination technology with great potential for removal and/or recycling ions from a range of waters. However, it still suffers from inefficient charge transfer and ion transport kinetics due to weak turbulence and low electric intensity in flow electrodes, both restricted by the current collectors. Herein, a new tip-array current collector (designated as T-CC) was developed to replace the conventional planar current collectors, which intensifies both the charge transfer and ion transport significantly. The effects of tip arrays on flow and electric fields were studied by both computational simulations and electrochemical impedance spectroscopy, which revealed the reduction of ion transport barrier, charge transport barrier and internal resistance. With the voltage increased from 1.0 to 1.5 and 2.0 V, the T-CC-based FCDI system (T-FCDI) exhibited average salt removal rates (ASRR) of 0.18, 0.50, and 0.89 μmol cm−2 min−1, respectively, which are 1.82, 2.65, and 2.48 folds higher than that of the conventional serpentine current collectors, and 1.48, 1.67, and 1.49 folds higher than that of the planar current collectors. Meanwhile, with the solid content in flow electrodes increased from 1 to 5 wt%, the ASRR for T-FCDI increased from 0.29 to 0.50 μmol cm−2 min−1, which are 1.70 and 1.67 folds higher than that of the planar current collectors. Additionally, a salt removal efficiency of 99.89% was achieved with T-FCDI and the charge efficiency remained above 95% after 24 h of operation, thus showing its superior long-term stability.
Highlights:
1 Steel tip arrays were used as current collectors to replace planar conductors.
2 Optimal flow and electric fields reduced barriers for electron and ion transport.
3 Desalination performance of flow-electrode capacitive deionization is enhanced by the tip-array current collectors.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- M. Elimelech, W.A. Phillip, The future of seawater desalination: energy, technology, and the environment. Science 333, 712–717 (2011). https://doi.org/10.1126/science.1200488
- Q. Dang, W. Zhang, J. Liu, L. Wang, D. Wu et al., Bias-free driven ion assisted photoelectrochemical system for sustainable wastewater treatment. Nat. Commun. 14, 8413 (2023). https://doi.org/10.1038/s41467-023-44155-5
- Q. Dang, L. Wang, W. Sun, G. Wu, S. Li et al., Solar-driven ion confinement for synergetic pollutants remediation and valuable metal recovery in wastewater. ACS ES&T Engg. 4, 1748–1757 (2024). https://doi.org/10.1021/acsestengg.4c00092
- W. Liu, Joshua L. Livingston, L. Wang et al., Pressure-driven membrane desalination. Nat. Rev. Methods Primers 4(1), 10 (2024). https://doi.org/10.1038/s43586-024-00296-5
- J. Eke, A. Yusuf, A. Giwa, A. Sodiq, The global status of desalination: an assessment of current desalination technologies, plants and capacity. Desalination 495, 114633 (2020). https://doi.org/10.1016/j.desal.2020.114633
- J. Liu, Q. Dang, L. Wang, D. Wang, L. Tang, Applications of flexible electrochemical electrodes in wastewater treatment: a review. Chin. Chem. Lett. 35, 109277 (2024). https://doi.org/10.1016/j.cclet.2023.109277
- Q. Dang, Y. Li, W. Zhang, Y.V. Kaneti, M. Hu et al., Spatial-controlled etching of coordination polymers. Chin. Chem. Lett. 32, 635–641 (2021). https://doi.org/10.1016/j.cclet.2020.04.053
- M.E. Suss, S. Porada, X. Sun, P.M. Biesheuvel, J. Yoon et al., Water desalination via capacitive deionization: what is it and what can we expect from it? Energy Environ. Sci. 8, 2296–2319 (2015). https://doi.org/10.1039/C5EE00519A
- J. Ma, S. Xing, Y. Wang, J. Yang, F. Yu, Kinetic-thermodynamic promotion engineering toward high-density hierarchical and Zn-doping activity-enhancing ZnNiO@CF for high-capacity desalination. Nano-Micro Lett. 16, 143 (2024). https://doi.org/10.1007/s40820-024-01371-y
- S. Porada, R. Zhao, A. van der Wal, V. Presser, P.M. Biesheuvel, Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 58, 1388–1442 (2013). https://doi.org/10.1016/j.pmatsci.2013.03.005
- H. Deng, W. Wei, L. Yao, Z. Zheng, B. Li et al., Potential-mediated recycling of copper from brackish water by an electrochemical copper pump. Adv. Sci. 9, e2203189 (2022). https://doi.org/10.1002/advs.202203189
- H. Deng, Z. Wang, M. Kim, Y. Yamauchi, S.J. Eichhorn et al., Unleashing the power of capacitive deionization: advancing ion removal with biomass-derived porous carbonaceous electrodes. Nano Energy 117, 108914 (2023). https://doi.org/10.1016/j.nanoen.2023.108914
- S.-I. Jeon, H.-R. Park, J.-G. Yeo, S. Yang, C.H. Cho et al., Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 6, 1471 (2013). https://doi.org/10.1039/c3ee24443a
- C. He, J. Ma, C. Zhang, J. Song, T.D. Waite, Short-circuited closed-cycle operation of flow-electrode CDI for brackish water softening. Environ. Sci. Technol. 52, 9350–9360 (2018). https://doi.org/10.1021/acs.est.8b02807
- H. Yin, L. Liu, J. Ma, C. Zhang, G. Qiu, Efficient removal of As(III) from groundwaters through self-alkalization in an asymmetric flow-electrode electrochemical separation system. Water Res. 246, 120734 (2023). https://doi.org/10.1016/j.watres.2023.120734
- C. Zhang, M. Wang, W. Xiao, J. Ma, J. Sun et al., Phosphate selective recovery by magnetic iron oxide impregnated carbon flow-electrode capacitive deionization (FCDI). Water Res. 189, 116653 (2021). https://doi.org/10.1016/j.watres.2020.116653
- K. Luo, Q. Niu, Y. Zhu, B. Song, G. Zeng et al., Desalination behavior and performance of flow-electrode capacitive deionization under various operational modes. Chem. Eng. J. 389, 124051 (2020). https://doi.org/10.1016/j.cej.2020.124051
- F. Yang, Y. He, L. Rosentsvit, M.E. Suss, X. Zhang et al., Flow-electrode capacitive deionization: a review and new perspectives. Water Res. 200, 117222 (2021). https://doi.org/10.1016/j.watres.2021.117222
- F. Yang, J. Ma, X. Zhang, X. Huang, P. Liang, Decreased charge transport distance by titanium mesh-membrane assembly for flow-electrode capacitive deionization with high desalination performance. Water Res. 164, 114904 (2019). https://doi.org/10.1016/j.watres.2019.114904
- J. Xiong, W. Ye, B. Xu, L. Mu, X. Lu et al., Quantitative decoupling of diffusion and electromigration of ions across membranes in flow capacitive deionization device. Ind. Eng. Chem. Res. 62, 14997–15005 (2023). https://doi.org/10.1021/acs.iecr.3c02210
- X. Zhang, M. Pang, Y. Wei, F. Liu, H. Zhang et al., Three-dimensional titanium mesh-based flow electrode capacitive deionization for salt separation and enrichment in high salinity water. Water Res. 251, 121147 (2024). https://doi.org/10.1016/j.watres.2024.121147
- C. Zhai, J. Yuan, Y. Wang, S. Liang, L. Yao et al., Rectorite in flow-electrode capacitive deionization with three-dimensional current collector to achieve cost-effective desalination. Desalination 573, 117217 (2024). https://doi.org/10.1016/j.desal.2023.117217
- X. Zhang, H. Zhou, Z. He, H. Zhang, H. Zhao, Flow-electrode capacitive deionization utilizing three-dimensional foam current collector for real seawater desalination. Water Res. 220, 118642 (2022). https://doi.org/10.1016/j.watres.2022.118642
- X. Zhang, J. Zhou, H. Zhou, H. Zhang, H. Zhao, Enhanced desalination performance by a novel Archimedes spiral flow channel for flow-electrode capacitive deionization. ACS ES&T Engg. 2, 1250–1259 (2022). https://doi.org/10.1021/acsestengg.1c00445
- J. Ma, X. Wang, Y. Bian, C. Zhang, C. Liu et al., Novel current collector with mosquito-repellent incense-shaped channel of flow electrode capacitive deionization. ACS Sustainable Chem. Eng. 10, 4818–4821 (2022). https://doi.org/10.1021/acssuschemeng.2c00442
- X. He, W. Chen, F. Sun, Z. Jiang, B. Li et al., Enhanced NH4+ removal and recovery from wastewater using Na-zeolite-based flow-electrode capacitive deionization: insight from ion transport flux. Environ. Sci. Technol. 57, 8828–8838 (2023). https://doi.org/10.1021/acs.est.3c02286
- L. Yan, J. Annor Asare, B. Wu, H. Gang, D. Wei et al., A hexagonal honeycomb-shaped flow channel for high-efficient desalination and flowability in flow-electrode capacitive deionization. ACS EST Water 3, 2753–2764 (2023). https://doi.org/10.1021/acsestwater.3c00242
- R. Chen, X. Deng, C. Wang, J. Du, Z. Zhao et al., A newly designed graphite-polyaniline composite current collector to enhance the performance of flow electrode capacitive deionization. Chem. Eng. J. 435, 134845 (2022). https://doi.org/10.1016/j.cej.2022.134845
- J. Zhou, Y.-C. Hung, X. Xie, Making waves: pathogen inactivation by electric field treatment: from liquid food to drinking water. Water Res. 207, 117817 (2021). https://doi.org/10.1016/j.watres.2021.117817
- T. Wang, X. Xie, Nanosecond bacteria inactivation realized by locally enhanced electric field treatment. Nat. Water 1, 104–112 (2023). https://doi.org/10.1038/s44221-022-00003-2
- M. Liu, Y. Pang, B. Zhang, P. De Luna, O. Voznyy et al., Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537, 382–386 (2016). https://doi.org/10.1038/nature19060
- S. Wang, J. Zhang, O. Gharbi, V. Vivier, M. Gao et al., Electrochemical impedance spectroscopy. Nat. Rev. Meth. Primers 1, 41 (2021). https://doi.org/10.1038/s43586-021-00039-w
- T.H. Wan, M. Saccoccio, C. Chen, F. Ciucci, Influence of the discretization methods on the distribution of relaxation times deconvolution: implementing radial basis functions with DRTtools. Electrochim. Acta 184, 483–499 (2015). https://doi.org/10.1016/j.electacta.2015.09.097
- S. COMSOL AB, Sweden. COMSOL Multiphysics® v. 6.1. cn.comsol.com. (2023).
- Y. Li, C. Ma, F. Qin, H. Chen, X. Zhao et al., The microstructure and mechanical properties of 316L austenitic stainless steel prepared by forge and laser melting deposition. Mater. Sci. Eng. A 870, 144820 (2023). https://doi.org/10.1016/j.msea.2023.144820
- V. Girault, P.-A. Raviart, Finite element methods for Navier-stokes equations (Springer, Berlin Heidelberg, 1986). https://doi.org/10.1007/978-3-642-61623-5
- A. Carmona-Orbezo, R.A.W. Dryfe, Understanding the performance of flow-electrodes for capacitive deionization through hydrodynamic voltammetry. Chem. Eng. J. 406, 126826 (2021). https://doi.org/10.1016/j.cej.2020.126826
- F. Summer, V. Zadin, S.S. Nakshatharan, A. Aabloo, J. Torop, Optimization of electrochemical flow capacitor (EFC) design via finite element modeling. J. Energy Storage 29, 101304 (2020). https://doi.org/10.1016/j.est.2020.101304
- R. Kafafy, T. Lin, Y. Lin, J. Wang, Three-dimensional immersed finite element methods for electric field simulation in composite materials. Int. J. Numer. Meth. Eng. 64, 940–972 (2005). https://doi.org/10.1002/nme.1401
- N. Kim, J. Park, Y. Cho, C.-Y. Yoo, Comprehensive electrochemical impedance spectroscopy study of flow-electrode capacitive deionization cells. Environ. Sci. Technol. 57, 8808–8817 (2023). https://doi.org/10.1021/acs.est.3c01619
- J. Guo, P. Brimley, M.J. Liu, E.R. Corson, C. Muñoz et al., Mass transport modifies the interfacial electrolyte to influence electrochemical nitrate reduction. ACS Sustain. Chem. Eng. 11, 7882–7893 (2023). https://doi.org/10.1021/acssuschemeng.3c01057
- Y. Tanaka, Concentration polarization in ion-exchange membrane electrodialysis. The events arising in an unforced flowing solution in a desalting cell. J. Membr. Sci. 244, 1–16 (2004). https://doi.org/10.1016/j.memsci.2004.02.041
- M.A. Osipenko, J. Karczewski, M. Dominów, M. Prześniak-Welenc, I.V. Makarava et al., Multisine impedimetric monitoring with an in-depth distribution of relaxation times analysis of WE43 and AZ31 magnesium alloys corrosion. Measurement 222, 113683 (2023). https://doi.org/10.1016/j.measurement.2023.113683
- Y. Li, Y. Jiang, J. Dang, X. Deng, B. Liu et al., Application of distribution of relaxation times method in polymer electrolyte membrane water electrolyzer. Chem. Eng. J. 451, 138327 (2023). https://doi.org/10.1016/j.cej.2022.138327
- S. Chandrasekaran, R. Hu, L. Yao, L. Sui, Y. Liu et al., Mutual self-regulation of d-electrons of single atoms and adjacent nanops for bifunctional oxygen electrocatalysis and rechargeable zinc-air batteries. Nano-Micro Lett. 15, 48 (2023). https://doi.org/10.1007/s40820-023-01022-8
- A. Rommerskirchen, B. Ohs, K.A. Hepp, R. Femmer, M. Wessling, Modeling continuous flow-electrode capacitive deionization processes with ion-exchange membranes. J. Membr. Sci. 546, 188–196 (2018). https://doi.org/10.1016/j.memsci.2017.10.026
- P.M. Biesheuvel, Y. Fu, M.Z. Bazant, Diffuse charge and Faradaic reactions in porous electrodes. Phys Rev. E Stat. Nonlin. Soft Matter Phys. 83, 061507 (2011). https://doi.org/10.1103/PhysRevE.83.061507
- P.M. Biesheuvel, S. Porada, M. Levi, M.Z. Bazant, Attractive forces in microporous carbon electrodes for capacitive deionization. J. Solid State Electrochem. 18, 1365–1376 (2014). https://doi.org/10.1007/s10008-014-2383-5
- K. Tang, S. Yiacoumi, Y. Li, J. Gabitto, C. Tsouris, Optimal conditions for efficient flow-electrode capacitive deionization. Sep. Purif. Technol. 240, 116626 (2020). https://doi.org/10.1016/j.seppur.2020.116626
- J. Ma, C. He, D. He, C. Zhang, T.D. Waite, Analysis of capacitive and electrodialytic contributions to water desalination by flow-electrode CDI. Water Res. 144, 296–303 (2018). https://doi.org/10.1016/j.watres.2018.07.049
- W. H. Organization, Guidelines for drinking-water quality: fourth edition incorporating first addendum. (World Health Organization; Geneva, 2017).
- H. Kariman, A. Shafieian, M. Khiadani, Small scale desalination technologies: a comprehensive review. Desalination 567, 116985 (2023). https://doi.org/10.1016/j.desal.2023.116985
- J. Ma, J. Ma, C. Zhang, J. Song, W. Dong et al., Flow-electrode capacitive deionization (FCDI) scale-up using a membrane stack configuration. Water Res. 168, 115186 (2020). https://doi.org/10.1016/j.watres.2019.115186
- N. Köller, L. Mankertz, S. Finger, C.J. Linnartz, M. Wessling, Towards pilot scale flow-electrode capacitive deionization. Desalination 572, 117096 (2024). https://doi.org/10.1016/j.desal.2023.117096
References
M. Elimelech, W.A. Phillip, The future of seawater desalination: energy, technology, and the environment. Science 333, 712–717 (2011). https://doi.org/10.1126/science.1200488
Q. Dang, W. Zhang, J. Liu, L. Wang, D. Wu et al., Bias-free driven ion assisted photoelectrochemical system for sustainable wastewater treatment. Nat. Commun. 14, 8413 (2023). https://doi.org/10.1038/s41467-023-44155-5
Q. Dang, L. Wang, W. Sun, G. Wu, S. Li et al., Solar-driven ion confinement for synergetic pollutants remediation and valuable metal recovery in wastewater. ACS ES&T Engg. 4, 1748–1757 (2024). https://doi.org/10.1021/acsestengg.4c00092
W. Liu, Joshua L. Livingston, L. Wang et al., Pressure-driven membrane desalination. Nat. Rev. Methods Primers 4(1), 10 (2024). https://doi.org/10.1038/s43586-024-00296-5
J. Eke, A. Yusuf, A. Giwa, A. Sodiq, The global status of desalination: an assessment of current desalination technologies, plants and capacity. Desalination 495, 114633 (2020). https://doi.org/10.1016/j.desal.2020.114633
J. Liu, Q. Dang, L. Wang, D. Wang, L. Tang, Applications of flexible electrochemical electrodes in wastewater treatment: a review. Chin. Chem. Lett. 35, 109277 (2024). https://doi.org/10.1016/j.cclet.2023.109277
Q. Dang, Y. Li, W. Zhang, Y.V. Kaneti, M. Hu et al., Spatial-controlled etching of coordination polymers. Chin. Chem. Lett. 32, 635–641 (2021). https://doi.org/10.1016/j.cclet.2020.04.053
M.E. Suss, S. Porada, X. Sun, P.M. Biesheuvel, J. Yoon et al., Water desalination via capacitive deionization: what is it and what can we expect from it? Energy Environ. Sci. 8, 2296–2319 (2015). https://doi.org/10.1039/C5EE00519A
J. Ma, S. Xing, Y. Wang, J. Yang, F. Yu, Kinetic-thermodynamic promotion engineering toward high-density hierarchical and Zn-doping activity-enhancing ZnNiO@CF for high-capacity desalination. Nano-Micro Lett. 16, 143 (2024). https://doi.org/10.1007/s40820-024-01371-y
S. Porada, R. Zhao, A. van der Wal, V. Presser, P.M. Biesheuvel, Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 58, 1388–1442 (2013). https://doi.org/10.1016/j.pmatsci.2013.03.005
H. Deng, W. Wei, L. Yao, Z. Zheng, B. Li et al., Potential-mediated recycling of copper from brackish water by an electrochemical copper pump. Adv. Sci. 9, e2203189 (2022). https://doi.org/10.1002/advs.202203189
H. Deng, Z. Wang, M. Kim, Y. Yamauchi, S.J. Eichhorn et al., Unleashing the power of capacitive deionization: advancing ion removal with biomass-derived porous carbonaceous electrodes. Nano Energy 117, 108914 (2023). https://doi.org/10.1016/j.nanoen.2023.108914
S.-I. Jeon, H.-R. Park, J.-G. Yeo, S. Yang, C.H. Cho et al., Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 6, 1471 (2013). https://doi.org/10.1039/c3ee24443a
C. He, J. Ma, C. Zhang, J. Song, T.D. Waite, Short-circuited closed-cycle operation of flow-electrode CDI for brackish water softening. Environ. Sci. Technol. 52, 9350–9360 (2018). https://doi.org/10.1021/acs.est.8b02807
H. Yin, L. Liu, J. Ma, C. Zhang, G. Qiu, Efficient removal of As(III) from groundwaters through self-alkalization in an asymmetric flow-electrode electrochemical separation system. Water Res. 246, 120734 (2023). https://doi.org/10.1016/j.watres.2023.120734
C. Zhang, M. Wang, W. Xiao, J. Ma, J. Sun et al., Phosphate selective recovery by magnetic iron oxide impregnated carbon flow-electrode capacitive deionization (FCDI). Water Res. 189, 116653 (2021). https://doi.org/10.1016/j.watres.2020.116653
K. Luo, Q. Niu, Y. Zhu, B. Song, G. Zeng et al., Desalination behavior and performance of flow-electrode capacitive deionization under various operational modes. Chem. Eng. J. 389, 124051 (2020). https://doi.org/10.1016/j.cej.2020.124051
F. Yang, Y. He, L. Rosentsvit, M.E. Suss, X. Zhang et al., Flow-electrode capacitive deionization: a review and new perspectives. Water Res. 200, 117222 (2021). https://doi.org/10.1016/j.watres.2021.117222
F. Yang, J. Ma, X. Zhang, X. Huang, P. Liang, Decreased charge transport distance by titanium mesh-membrane assembly for flow-electrode capacitive deionization with high desalination performance. Water Res. 164, 114904 (2019). https://doi.org/10.1016/j.watres.2019.114904
J. Xiong, W. Ye, B. Xu, L. Mu, X. Lu et al., Quantitative decoupling of diffusion and electromigration of ions across membranes in flow capacitive deionization device. Ind. Eng. Chem. Res. 62, 14997–15005 (2023). https://doi.org/10.1021/acs.iecr.3c02210
X. Zhang, M. Pang, Y. Wei, F. Liu, H. Zhang et al., Three-dimensional titanium mesh-based flow electrode capacitive deionization for salt separation and enrichment in high salinity water. Water Res. 251, 121147 (2024). https://doi.org/10.1016/j.watres.2024.121147
C. Zhai, J. Yuan, Y. Wang, S. Liang, L. Yao et al., Rectorite in flow-electrode capacitive deionization with three-dimensional current collector to achieve cost-effective desalination. Desalination 573, 117217 (2024). https://doi.org/10.1016/j.desal.2023.117217
X. Zhang, H. Zhou, Z. He, H. Zhang, H. Zhao, Flow-electrode capacitive deionization utilizing three-dimensional foam current collector for real seawater desalination. Water Res. 220, 118642 (2022). https://doi.org/10.1016/j.watres.2022.118642
X. Zhang, J. Zhou, H. Zhou, H. Zhang, H. Zhao, Enhanced desalination performance by a novel Archimedes spiral flow channel for flow-electrode capacitive deionization. ACS ES&T Engg. 2, 1250–1259 (2022). https://doi.org/10.1021/acsestengg.1c00445
J. Ma, X. Wang, Y. Bian, C. Zhang, C. Liu et al., Novel current collector with mosquito-repellent incense-shaped channel of flow electrode capacitive deionization. ACS Sustainable Chem. Eng. 10, 4818–4821 (2022). https://doi.org/10.1021/acssuschemeng.2c00442
X. He, W. Chen, F. Sun, Z. Jiang, B. Li et al., Enhanced NH4+ removal and recovery from wastewater using Na-zeolite-based flow-electrode capacitive deionization: insight from ion transport flux. Environ. Sci. Technol. 57, 8828–8838 (2023). https://doi.org/10.1021/acs.est.3c02286
L. Yan, J. Annor Asare, B. Wu, H. Gang, D. Wei et al., A hexagonal honeycomb-shaped flow channel for high-efficient desalination and flowability in flow-electrode capacitive deionization. ACS EST Water 3, 2753–2764 (2023). https://doi.org/10.1021/acsestwater.3c00242
R. Chen, X. Deng, C. Wang, J. Du, Z. Zhao et al., A newly designed graphite-polyaniline composite current collector to enhance the performance of flow electrode capacitive deionization. Chem. Eng. J. 435, 134845 (2022). https://doi.org/10.1016/j.cej.2022.134845
J. Zhou, Y.-C. Hung, X. Xie, Making waves: pathogen inactivation by electric field treatment: from liquid food to drinking water. Water Res. 207, 117817 (2021). https://doi.org/10.1016/j.watres.2021.117817
T. Wang, X. Xie, Nanosecond bacteria inactivation realized by locally enhanced electric field treatment. Nat. Water 1, 104–112 (2023). https://doi.org/10.1038/s44221-022-00003-2
M. Liu, Y. Pang, B. Zhang, P. De Luna, O. Voznyy et al., Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537, 382–386 (2016). https://doi.org/10.1038/nature19060
S. Wang, J. Zhang, O. Gharbi, V. Vivier, M. Gao et al., Electrochemical impedance spectroscopy. Nat. Rev. Meth. Primers 1, 41 (2021). https://doi.org/10.1038/s43586-021-00039-w
T.H. Wan, M. Saccoccio, C. Chen, F. Ciucci, Influence of the discretization methods on the distribution of relaxation times deconvolution: implementing radial basis functions with DRTtools. Electrochim. Acta 184, 483–499 (2015). https://doi.org/10.1016/j.electacta.2015.09.097
S. COMSOL AB, Sweden. COMSOL Multiphysics® v. 6.1. cn.comsol.com. (2023).
Y. Li, C. Ma, F. Qin, H. Chen, X. Zhao et al., The microstructure and mechanical properties of 316L austenitic stainless steel prepared by forge and laser melting deposition. Mater. Sci. Eng. A 870, 144820 (2023). https://doi.org/10.1016/j.msea.2023.144820
V. Girault, P.-A. Raviart, Finite element methods for Navier-stokes equations (Springer, Berlin Heidelberg, 1986). https://doi.org/10.1007/978-3-642-61623-5
A. Carmona-Orbezo, R.A.W. Dryfe, Understanding the performance of flow-electrodes for capacitive deionization through hydrodynamic voltammetry. Chem. Eng. J. 406, 126826 (2021). https://doi.org/10.1016/j.cej.2020.126826
F. Summer, V. Zadin, S.S. Nakshatharan, A. Aabloo, J. Torop, Optimization of electrochemical flow capacitor (EFC) design via finite element modeling. J. Energy Storage 29, 101304 (2020). https://doi.org/10.1016/j.est.2020.101304
R. Kafafy, T. Lin, Y. Lin, J. Wang, Three-dimensional immersed finite element methods for electric field simulation in composite materials. Int. J. Numer. Meth. Eng. 64, 940–972 (2005). https://doi.org/10.1002/nme.1401
N. Kim, J. Park, Y. Cho, C.-Y. Yoo, Comprehensive electrochemical impedance spectroscopy study of flow-electrode capacitive deionization cells. Environ. Sci. Technol. 57, 8808–8817 (2023). https://doi.org/10.1021/acs.est.3c01619
J. Guo, P. Brimley, M.J. Liu, E.R. Corson, C. Muñoz et al., Mass transport modifies the interfacial electrolyte to influence electrochemical nitrate reduction. ACS Sustain. Chem. Eng. 11, 7882–7893 (2023). https://doi.org/10.1021/acssuschemeng.3c01057
Y. Tanaka, Concentration polarization in ion-exchange membrane electrodialysis. The events arising in an unforced flowing solution in a desalting cell. J. Membr. Sci. 244, 1–16 (2004). https://doi.org/10.1016/j.memsci.2004.02.041
M.A. Osipenko, J. Karczewski, M. Dominów, M. Prześniak-Welenc, I.V. Makarava et al., Multisine impedimetric monitoring with an in-depth distribution of relaxation times analysis of WE43 and AZ31 magnesium alloys corrosion. Measurement 222, 113683 (2023). https://doi.org/10.1016/j.measurement.2023.113683
Y. Li, Y. Jiang, J. Dang, X. Deng, B. Liu et al., Application of distribution of relaxation times method in polymer electrolyte membrane water electrolyzer. Chem. Eng. J. 451, 138327 (2023). https://doi.org/10.1016/j.cej.2022.138327
S. Chandrasekaran, R. Hu, L. Yao, L. Sui, Y. Liu et al., Mutual self-regulation of d-electrons of single atoms and adjacent nanops for bifunctional oxygen electrocatalysis and rechargeable zinc-air batteries. Nano-Micro Lett. 15, 48 (2023). https://doi.org/10.1007/s40820-023-01022-8
A. Rommerskirchen, B. Ohs, K.A. Hepp, R. Femmer, M. Wessling, Modeling continuous flow-electrode capacitive deionization processes with ion-exchange membranes. J. Membr. Sci. 546, 188–196 (2018). https://doi.org/10.1016/j.memsci.2017.10.026
P.M. Biesheuvel, Y. Fu, M.Z. Bazant, Diffuse charge and Faradaic reactions in porous electrodes. Phys Rev. E Stat. Nonlin. Soft Matter Phys. 83, 061507 (2011). https://doi.org/10.1103/PhysRevE.83.061507
P.M. Biesheuvel, S. Porada, M. Levi, M.Z. Bazant, Attractive forces in microporous carbon electrodes for capacitive deionization. J. Solid State Electrochem. 18, 1365–1376 (2014). https://doi.org/10.1007/s10008-014-2383-5
K. Tang, S. Yiacoumi, Y. Li, J. Gabitto, C. Tsouris, Optimal conditions for efficient flow-electrode capacitive deionization. Sep. Purif. Technol. 240, 116626 (2020). https://doi.org/10.1016/j.seppur.2020.116626
J. Ma, C. He, D. He, C. Zhang, T.D. Waite, Analysis of capacitive and electrodialytic contributions to water desalination by flow-electrode CDI. Water Res. 144, 296–303 (2018). https://doi.org/10.1016/j.watres.2018.07.049
W. H. Organization, Guidelines for drinking-water quality: fourth edition incorporating first addendum. (World Health Organization; Geneva, 2017).
H. Kariman, A. Shafieian, M. Khiadani, Small scale desalination technologies: a comprehensive review. Desalination 567, 116985 (2023). https://doi.org/10.1016/j.desal.2023.116985
J. Ma, J. Ma, C. Zhang, J. Song, W. Dong et al., Flow-electrode capacitive deionization (FCDI) scale-up using a membrane stack configuration. Water Res. 168, 115186 (2020). https://doi.org/10.1016/j.watres.2019.115186
N. Köller, L. Mankertz, S. Finger, C.J. Linnartz, M. Wessling, Towards pilot scale flow-electrode capacitive deionization. Desalination 572, 117096 (2024). https://doi.org/10.1016/j.desal.2023.117096